Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system
- PMID: 17298183
- PMCID: PMC1808500
- DOI: 10.1371/journal.pbio.0050039
Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system
Abstract
Support of ageing neurons by endogenous neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) may determine whether the neurons resist or succumb to neurodegeneration. GDNF has been tested in clinical trials for the treatment of Parkinson disease (PD), a common neurodegenerative disorder characterized by the loss of midbrain dopaminergic (DA) neurons. BDNF modulates nigrostriatal functions and rescues DA neurons in PD animal models. The physiological roles of GDNF and BDNF signaling in the adult nigrostriatal DA system are unknown. We generated mice with regionally selective ablations of the genes encoding the receptors for GDNF (Ret) and BDNF (TrkB). We find that Ret, but not TrkB, ablation causes progressive and adult-onset loss of DA neurons specifically in the substantia nigra pars compacta, degeneration of DA nerve terminals in striatum, and pronounced glial activation. These findings establish Ret as a critical regulator of long-term maintenance of the nigrostriatal DA system and suggest conditional Ret mutants as useful tools for gaining insights into the molecular mechanisms involved in the development of PD.
Conflict of interest statement
Figures
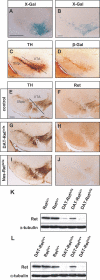





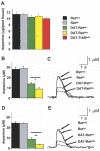
References
-
- Forman MS, Lee VM, Trojanowski JQ. Nosology of Parkinson's disease: Looking for the way out of a quagmire. Neuron. 2005;47:479–482. - PubMed
-
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S49–S58. - PubMed
-
- Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci. 2003;26:215–221. - PubMed
-
- Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. - PubMed
-
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

