A novel role for the TIR domain in association with pathogen-derived elicitors
- PMID: 17298188
- PMCID: PMC1820829
- DOI: 10.1371/journal.pbio.0050068
A novel role for the TIR domain in association with pathogen-derived elicitors
Erratum in
-
Correction: A Novel Role for the TIR Domain in Association with Pathogen-Derived Elicitors.PLoS Biol. 2016 Jan 25;14(1):e1002374. doi: 10.1371/journal.pbio.1002374. eCollection 2016 Jan. PLoS Biol. 2016. PMID: 26807877 Free PMC article.
Abstract
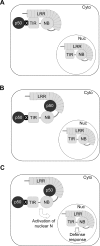
Plant innate immunity is mediated by Resistance (R) proteins, which bear a striking resemblance to animal molecules of similar function. Tobacco N is a TIR-NB-LRR R gene that confers resistance to Tobacco mosaic virus, specifically the p50 helicase domain. An intriguing question is how plant R proteins recognize the presence of pathogen-derived Avirulence (Avr) elicitor proteins. We have used biochemical cell fraction and immunoprecipitation in addition to confocal fluorescence microscopy of living tissue to examine the association between N and p50. Surprisingly, both N and p50 are cytoplasmic and nuclear proteins, and N's nuclear localization is required for its function. We also demonstrate an in planta association between N and p50. Further, we show that N's TIR domain is critical for this association, and indeed, it alone can associate with p50. Our results differ from current models for plant innate immunity that propose detection is mediated solely through the LRR domains of these molecules. The data we present support an intricate process of pathogen elicitor recognition by R proteins involving multiple subcellular compartments and the formation of multiple protein complexes.
Conflict of interest statement
Figures
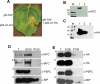
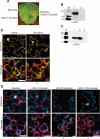
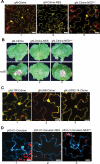
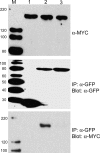
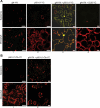
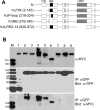
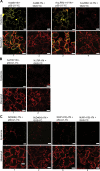
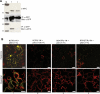
References
-
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. - PubMed
-
- Flor H. Current status of the gene-for-gene concept. Annu Rev Phytopathol. 1971;9:275–296.
-
- Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. - PubMed
-
- Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. - PubMed
-
- van der Biezen EA, Jones JD. The NB-ARC domain: A novel signaling motif shared by plant resistance gene products and regulators of cell death in animals. Curr Biol. 1998;8:R226–R227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

