The major determinant of the heparin binding of glial cell-line-derived neurotrophic factor is near the N-terminus and is dispensable for receptor binding
- PMID: 17298301
- PMCID: PMC1868828
- DOI: 10.1042/BJ20061747
The major determinant of the heparin binding of glial cell-line-derived neurotrophic factor is near the N-terminus and is dispensable for receptor binding
Abstract
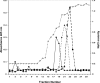
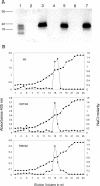
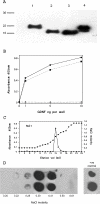
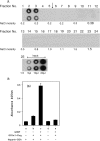
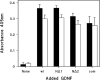
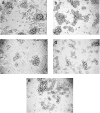
GDNF (glial cell-line-derived neurotrophic factor), and the closely related cytokines artemin and neurturin, bind strongly to heparin. Deletion of a basic amino-acid-rich sequence of 16 residues N-terminal to the first cysteine of the transforming growth factor beta domain of GDNF results in a marked reduction in heparin binding, whereas removal of a neighbouring sequence, and replacement of pairs of other basic residues with alanine had no effect. The heparin-binding sequence is quite distinct from the binding site for the high affinity GDNF polypeptide receptor, GFRalpha1 (GDNF family receptor alpha1), and heparin-bound GDNF is able to bind GFRalpha1 simultaneously. The heparin-binding sequence of GDNF is dispensable both for GFRalpha1 binding, and for activity for in vitro neurite outgrowth assay. Surprisingly, the observed inhibition of GDNF bioactivity with the wild-type protein in this assay was still found with the deletion mutant lacking the heparin-binding sequence. Heparin neither inhibits nor potentiates GDNF-GFRalpha1 interaction, and the extracellular domain of GFRalpha1 does not bind to heparin itself, precluding heparin cross-bridging of cytokine and receptor polypeptides. The role of heparin and heparan sulfate in GDNF signalling remains unclear, but the present study indicates that it does not occur in the first step of the pathway, namely GDNF-GFRalpha1 engagement.
Figures


References
-
- Baloh R., Enomoto H., Johnson E., Milbrandt J. The GDNF family ligands and receptors: implications for neural development. Curr. Opin. Neurobiol. 2000;10:103–110. - PubMed
-
- Carmillo P., Dago L., Day E., Worley D., Rossomando A., Walus L., Orozco O., Buckley C., Miller S., Tse A., et al. Glial cell line-derived neurotrophic factor (GDNF) receptor α-1 (GFRα1) is highly selective for GDNF versus artemin. Biochemistry. 2005;44:2545–2554. - PubMed
-
- Poteryaev D., Titievsky A., Sun Y. F., Thomas-Crusells J., Lindahl M., Billaud M., Arumae U., Saarma M. GDNF triggers a novel ret-independent Src kinase family-coupled signalling via a GPI-linked GDNF receptor α1. FEBS Lett. 1999;463:63–66. - PubMed
-
- Trupp M., Scott R., Whittlemore S. R., Ibanez C. F. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signalling in neuronal cells. J. Biol. Chem. 1999;274:20885–20894. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

