Relocalization of STIM1 for activation of store-operated Ca(2+) entry is determined by the depletion of subplasma membrane endoplasmic reticulum Ca(2+) store
- PMID: 17298947
- PMCID: PMC3309416
- DOI: 10.1074/jbc.M609435200
Relocalization of STIM1 for activation of store-operated Ca(2+) entry is determined by the depletion of subplasma membrane endoplasmic reticulum Ca(2+) store
Abstract
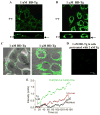
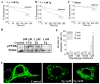
STIM1 (stromal interacting molecule 1), an endoplasmic reticulum (ER) protein that controls store-operated Ca(2+) entry (SOCE), redistributes into punctae at the cell periphery after store depletion. This redistribution is suggested to have a causal role in activation of SOCE. However, whether peripheral STIM1 punctae that are involved in regulation of SOCE are determined by depletion of peripheral or more internal ER has not yet been demonstrated. Here we show that Ca(2+) depletion in subplasma membrane ER is sufficient for peripheral redistribution of STIM1 and activation of SOCE. 1 microM thapsigargin (Tg) induced substantial depletion of intracellular Ca(2+) stores and rapidly activated SOCE. In comparison, 1 nM Tg induced slower, about 60-70% less Ca(2+) depletion but similar SOCE. SOCE was confirmed by measuring I(SOC) in addition to Ca(2+), Mn(2+), and Ba(2+) entry. Importantly, 1 nM Tg caused redistribution of STIM1 only in the ER-plasma membrane junction, whereas 1 microM Tg caused a relatively global relocalization of STIM1 in the cell. During the time taken for STIM1 relocalization and SOCE activation, 1 nM Bodipy-fluorescein Tg primarily labeled the subplasma membrane region, whereas 1 microM Tg labeled the entire cell. The localization of Tg in the subplasma membrane region was associated with depletion of ER in this region and activation of SOCE. Together, these data suggest that peripheral STIM1 relocalization that is causal in regulation of SOCE is determined by the status of [Ca(2+)] in the ER in close proximity to the plasma membrane. Thus, the mechanism involved in regulation of SOCE is contained within the ER-plasma membrane junctional region.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

