Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells
- PMID: 17299037
- PMCID: PMC1815273
- DOI: 10.1073/pnas.0611493104
Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells
Abstract
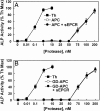
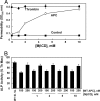
Ever-increasing evidence in the literature suggests that the antiinflammatory and cytoprotective properties of activated protein C (APC) are mediated through its endothelial protein C receptor (EPCR)-dependent cleavage of protease-activated receptor 1 (PAR-1) on endothelial cells. However, recent results monitoring the cleavage rate of PAR-1 on human umbilical vein endothelial cells, transfected with an alkaline phosphatase-PAR-1 fusion reporter construct, have indicated that the catalytic activity of thrombin toward PAR-1 is several orders of magnitude higher than that of APC. Because thrombin is required for generation of APC, and because it also functions in the proinflammatory pathways through the activation of PAR-1, it has been difficult to understand how APC can elicit protective cellular responses through the activation of PAR-1 when thrombin is present. In this study we provide a plausible answer to this question by demonstrating that the critical receptors required for both protein C activation (thrombomodulin and EPCR) and APC cellular signaling (EPCR and PAR-1) pathways are colocalized in the membrane lipid rafts in endothelial cells. We further show that the APC cleavage of PAR-1 on cells transfected with a PAR-1 cleavage reporter construct is not sensitive to the cofactor function of EPCR. Thus, the colocalization of EPCR and PAR-1 in lipid rafts is a key requirement for the cellular signaling activity of APC. Thrombomodulin colocalization with these receptors on the same membrane microdomain can also recruit thrombin to activate the EPCR-bound protein C, thereby eliciting PAR-1 signaling events that are involved in the APC protective pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

