The Fas pathway is involved in pancreatic beta cell secretory function
- PMID: 17299038
- PMCID: PMC1815272
- DOI: 10.1073/pnas.0611487104
The Fas pathway is involved in pancreatic beta cell secretory function
Abstract
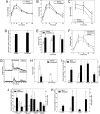
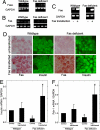
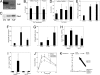
Pancreatic beta cell mass and function increase in conditions of enhanced insulin demand such as obesity. Failure to adapt leads to diabetes. The molecular mechanisms controlling this adaptive process are unclear. Fas is a death receptor involved in beta cell apoptosis or proliferation, depending on the activity of the caspase-8 inhibitor FLIP. Here we show that the Fas pathway also regulates beta cell secretory function. We observed impaired glucose tolerance in Fas-deficient mice due to a delayed and decreased insulin secretory pattern. Expression of PDX-1, a beta cell-specific transcription factor regulating insulin gene expression and mitochondrial metabolism, was decreased in Fas-deficient beta cells. As a consequence, insulin and ATP production were severely reduced and only partly compensated for by increased beta cell mass. Up-regulation of FLIP enhanced NF-kappaB activity via NF-kappaB-inducing kinase and RelB. This led to increased PDX-1 and insulin production independent of changes in cell turnover. The results support a previously undescribed role for the Fas pathway in regulating insulin production and release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
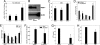
References
-
- Rhodes CJ. Science. 2005;307:380–384. - PubMed
-
- Donath MY, Halban PA. Diabetologia. 2004;47:581–589. - PubMed
-
- Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY. Diabetes. 2001;50:1683–1690. - PubMed
-
- Donath MY, Storling J, Maedler K, Mandrup-Poulsen T. J Mol Med. 2003;81:455–470. - PubMed
-
- Nolsoe RL, Hamid YH, Pociot F, Paulsen S, Andersen KM, Borch-Johnsen K, Drivsholm T, Hansen T, Pedersen O, Mandrup-Poulsen T. Genes Immun. 2006;7:316–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

