Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B
- PMID: 17299053
- PMCID: PMC1815279
- DOI: 10.1073/pnas.0608374104
Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B
Abstract
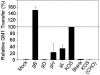
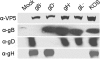
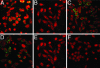
Virus-induced membrane fusion can be subdivided into three phases defined by studies of class I and class II fusion proteins. During Phase I, two membranes are brought into close apposition. Phase II marks the mixing of the outer membrane leaflets leading to formation of a hemifusion intermediate. A fusion pore stably forms and expands in Phase III, thereby completing the fusion process. Herpes simplex virus type 1 (HSV-1) requires four glycoproteins to complete membrane fusion, but none has been defined as class I or II. Therefore, we investigated whether HSV-1-induced membrane fusion occurred following the same general phases as those described for class I and II proteins. In this study we demonstrate that glycoprotein D (gD) and the glycoprotein H and glycoprotein L complex (gHL) mediated lipid mixing indicative of hemifusion. However, content mixing and full fusion required glycoprotein B (gB) to be present along with gD and gHL. Our results indicate that, like class I and II fusion proteins, fusion mediated by HSV-1 glycoproteins occurred through a hemifusion intermediate. In addition, both gB and gHL are probably directly involved in the fusion process. From this, we propose a sequential model for fusion via HSV-1 glycoproteins whereby gD is required for Phase I, gHL is required for Phase II, and gB is required for Phase III. We further propose that glycoprotein H and gB are likely to function sequentially to promote membrane fusion in other herpesviruses such as Epstein-Barr virus and human herpesvirus 8.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chernomordik LV, Kozlov MM. Cell. 2005;123:375–382. - PubMed
-
- Jardetzky TS, Lamb RA. Nature. 2004;427:307–308. - PubMed
-
- Blumenthal R, Clague MJ, Durell SR, Epand RM. Chem Rev. 2003;103:53–69. - PubMed
-
- Cohen FS, Melikyan GB. Methods. 1998;16:215–226. - PubMed
-
- Spear PG. Cell Microbiol. 2004;6:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

