Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin
- PMID: 17299088
- PMCID: PMC1885515
- DOI: 10.1182/blood-2006-09-048868
Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin
Abstract
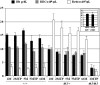
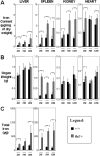
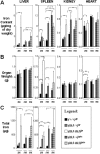
Progressive iron overload is the most salient and ultimately fatal complication of beta-thalassemia. However, little is known about the relationship among ineffective erythropoiesis (IE), the role of iron-regulatory genes, and tissue iron distribution in beta-thalassemia. We analyzed tissue iron content and iron-regulatory gene expression in the liver, duodenum, spleen, bone marrow, kidney, and heart of mice up to 1 year old that exhibit levels of iron overload and anemia consistent with both beta-thalassemia intermedia (th3/+) and major (th3/th3). Here we show, for the first time, that tissue and cellular iron distribution are abnormal and different in th3/+ and th3/th3 mice, and that transfusion therapy can rescue mice affected by beta-thalassemia major and modify both the absorption and distribution of iron. Our study reveals that the degree of IE dictates tissue iron distribution and that IE and iron content regulate hepcidin (Hamp1) and other iron-regulatory genes such as Hfe and Cebpa. In young th3/+ and th3/th3 mice, low Hamp1 levels are responsible for increased iron absorption. However, in 1-year-old th3/+ animals, Hamp1 levels rise and it is rather the increase of ferroportin (Fpn1) that sustains iron accumulation, thus revealing a fundamental role of this iron transporter in the iron overload of beta-thalassemia.
Figures
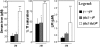

References
-
- Cooley TB, Lee P. A series of cases of splenomegaly in children with anemia and peculiar bone changes. Trans Am Pediat. Soc. 1925;37:29–33.
-
- Giardina PJ, Grady RW. Chelation therapy in beta-thalassemia: an optimistic update. Semin Hematol. 2001;38:360–366. - PubMed
-
- Bannerman RM, Keusch G, Kreimer-Birnbaum M, Vance VK, Vaughan S. Thalassemia intermedia, with iron overload, cardiac failure, diabetes mellitus, hypopituitarism and porphyrinuria. Am J Med. 1967;42:476–486. - PubMed
-
- Heinrich HC, Gabbe EE, Oppitz KH, et al. Absorption of inorganic and food iron in children with heterozygous and homozygous beta-thalassemia. Z Kinderheilkd. 1973;115:1–22. - PubMed
-
- Cossu P, Toccafondi C, Vardeu F, et al. Iron overload and desferrioxamine chelation therapy in beta-thalassemia intermedia. Eur J Pediatr. 1981;137:267–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

