Non-metabolic membrane tubulation and permeability induced by bioactive peptides
- PMID: 17299584
- PMCID: PMC1790702
- DOI: 10.1371/journal.pone.0000201
Non-metabolic membrane tubulation and permeability induced by bioactive peptides
Abstract
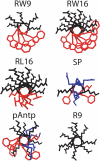
Background: Basic cell-penetrating peptides are potential vectors for therapeutic molecules and display antimicrobial activity. The peptide-membrane contact is the first step of the sequential processes leading to peptide internalization and cell activity. However, the molecular mechanisms involved in peptide-membrane interaction are not well understood and are frequently controversial. Herein, we compared the membrane activities of six basic peptides with different size, charge density and amphipaticity: Two cell-penetrating peptides (penetratin and R9), three amphipathic peptides and the neuromodulator substance P.
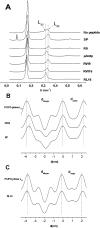
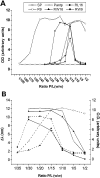
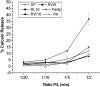
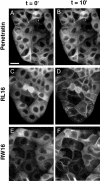
Methodology/principal findings: Experiments of X ray diffraction, video-microscopy of giant vesicles, fluorescence spectroscopy, turbidimetry and calcein leakage from large vesicles are reported. Permeability and toxicity experiments were performed on cultured cells. The peptides showed differences in bilayer thickness perturbations, vesicles aggregation and local bending properties which form lipidic tubular structures. These structures invade the vesicle lumen in the absence of exogenous energy.
Conclusions/significance: We showed that the degree of membrane permeabilization with amphipathic peptides is dependent on both peptide size and hydrophobic nature of the residues. We propose a model for peptide-induced membrane perturbations that explains the differences in peptide membrane activities and suggests the existence of a facilitated "physical endocytosis," which represents a new pathway for peptide cellular internalization.
Conflict of interest statement
Figures


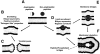
References
-
- Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. - PubMed
-
- Nekhotiaeva N, Elmquist A, Rajarao GK, Hallbrink M, Langel U, et al. Cell entry and antimicrobial properties of eukaryotic cell-penetrating peptides. Faseb J. 2004;18:394–396. - PubMed
-
- Palm C, Netzereab S, Hallbrink M. Quantitatively determined uptake of cell-penetrating peptides in non-mammalian cells with an evaluation of degradation and antimicrobial effects. Peptides. 2006;27:1710–1716. - PubMed
-
- Duplaa H, Convert O, Sautereau AM, Tocanne JF, Chassaing G. Binding of substance P to monolayers and vesicles made of phosphatidylcholine and/or phosphatidylserine. Biochim Biophys Acta. 1992;1107:12–22. - PubMed
-
- Oehlke J, Lorenz D, Wiesner B, Bienert M. Studies on the cellular uptake of substance P and lysine-rich, KLA-derived model peptides. J Mol Recognit. 2005;18:50–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

