Black tea prevents cigarette smoke-induced apoptosis and lung damage
- PMID: 17300721
- PMCID: PMC1802835
- DOI: 10.1186/1476-9255-4-3
Black tea prevents cigarette smoke-induced apoptosis and lung damage
Abstract
Background: Cigarette smoking is a major cause of lung damage. One prominent deleterious effect of cigarette smoke is oxidative stress. Oxidative stress may lead to apoptosis and lung injury. Since black tea has antioxidant property, we examined the preventive effect of black tea on cigarette smoke-induced oxidative damage, apoptosis and lung injury in a guinea pig model.
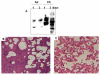
Methods: Guinea pigs were subjected to cigarette smoke exposure from five cigarettes (two puffs/cigarette) per guinea pig/day for seven days and given water or black tea to drink. Sham control guinea pigs were exposed to air instead of cigarette smoke. Lung damage, as evidenced by inflammation and increased air space, was assessed by histology and morphometric analysis. Protein oxidation was measured through oxyblot analysis of dinitrophenylhydrazone derivatives of the protein carbonyls of the oxidized proteins. Apoptosis was evidenced by the fragmentation of DNA using TUNEL assay, activation of caspase 3, phosphorylation of p53 as well as over-expression of Bax by immunoblot analyses.
Results: Cigarette smoke exposure to a guinea pig model caused lung damage. It appeared that oxidative stress was the initial event, which was followed by inflammation, apoptosis and lung injury. All these pathophysiological events were prevented when the cigarette smoke-exposed guinea pigs were given black tea infusion as the drink instead of water.
Conclusion: Cigarette smoke exposure to a guinea pig model causes oxidative damage, inflammation, apoptosis and lung injury that are prevented by supplementation of black tea.
Figures
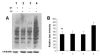




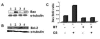
Similar articles
-
Cellular and molecular mechanisms of cigarette smoke-induced lung damage and prevention by vitamin C.J Inflamm (Lond). 2008 Nov 11;5:21. doi: 10.1186/1476-9255-5-21. J Inflamm (Lond). 2008. PMID: 19014449 Free PMC article.
-
Black tea prevents cigarette smoke-induced oxidative damage of proteins in guinea pigs.J Nutr. 2003 Aug;133(8):2622-8. doi: 10.1093/jn/133.8.2622. J Nutr. 2003. PMID: 12888648
-
Chinese green tea consumption reduces oxidative stress, inflammation and tissues damage in smoke exposed rats.Iran J Basic Med Sci. 2014 Oct;17(10):740-6. Iran J Basic Med Sci. 2014. PMID: 25729541 Free PMC article.
-
Vitamin C prevents cigarette smoke-induced oxidative damage in vivo.Free Radic Biol Med. 2000 Jul 15;29(2):115-24. doi: 10.1016/s0891-5849(00)00297-5. Free Radic Biol Med. 2000. PMID: 10980400
-
Vitamin C prevents cigarette smoke induced atherosclerosis in guinea pig model.J Atheroscler Thromb. 2010 Aug 31;17(8):817-27. doi: 10.5551/jat.2881. Epub 2010 May 13. J Atheroscler Thromb. 2010. PMID: 20467194
Cited by
-
Effects of passive inhalation of cigarette smoke on structural and functional parameters in the respiratory system of guinea pigs.J Bras Pneumol. 2016 Sep-Oct;42(5):333-340. doi: 10.1590/S1806-37562015000000342. J Bras Pneumol. 2016. PMID: 27812632 Free PMC article.
-
Pathobiology of cigarette smoke-induced invasive cancer of the renal pelvis and its prevention by vitamin C.Toxicol Rep. 2018 Oct 5;5:1002-1010. doi: 10.1016/j.toxrep.2018.10.005. eCollection 2018. Toxicol Rep. 2018. PMID: 30338226 Free PMC article.
-
Activated charcoal filter effectively reduces p-benzosemiquinone from the mainstream cigarette smoke and prevents emphysema.J Biosci. 2010 Jun;35(2):217-30. doi: 10.1007/s12038-010-0026-2. J Biosci. 2010. PMID: 20689178
-
Cellular and molecular mechanisms of cigarette smoke-induced lung damage and prevention by vitamin C.J Inflamm (Lond). 2008 Nov 11;5:21. doi: 10.1186/1476-9255-5-21. J Inflamm (Lond). 2008. PMID: 19014449 Free PMC article.
-
IKKβ-I-κBɛ-c-Rel/p50: a new axis of NF-κB activation in lung epithelial cells.Oncogenesis. 2012 Apr 9;1(4):e8. doi: 10.1038/oncsis.2012.8. Oncogenesis. 2012. PMID: 23552605 Free PMC article.
References
-
- Janoff A. Investigations into the biochemical mechanisms of pulmonary emphysema: effects of cigarette smoke on enzymes and anti-enzymes in the lung. Respiration. 1986;50:13–25. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

