Mitochondrial protein import and human health and disease
- PMID: 17300922
- PMCID: PMC2702852
- DOI: 10.1016/j.bbadis.2006.12.002
Mitochondrial protein import and human health and disease
Abstract
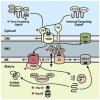
The targeting and assembly of nuclear-encoded mitochondrial proteins are essential processes because the energy supply of humans is dependent upon the proper functioning of mitochondria. Defective import of mitochondrial proteins can arise from mutations in the targeting signals within precursor proteins, from mutations that disrupt the proper functioning of the import machinery, or from deficiencies in the chaperones involved in the proper folding and assembly of proteins once they are imported. Defects in these steps of import have been shown to lead to oxidative stress, neurodegenerative diseases, and metabolic disorders. In addition, protein import into mitochondria has been found to be a dynamically regulated process that varies in response to conditions such as oxidative stress, aging, drug treatment, and exercise. This review focuses on how mitochondrial protein import affects human health and disease.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

