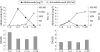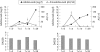Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis
- PMID: 17301106
- PMCID: PMC1955110
- DOI: 10.1136/ard.2006.065615
Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis
Abstract
Background: A substantial proportion of patients with rheumatoid arthritis (RA) do not respond, or lose initial response, to adalimumab treatment. One explanation for non-response is that patients develop anti-adalimumab antibodies.
Objectives: To evaluate the incidence of formation of antibody against adalimumab and the association with serum adalimumab concentrations and clinical response.
Methods: In a cohort of 121 consecutive patients with RA treated with adalimumab, serum adalimumab concentrations and antibodies against adalimumab were measured together with clinical response variables before and up to 28 weeks after the start of treatment.
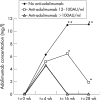
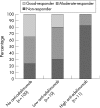
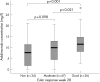
Results: Anti-adalimumab antibodies were detected in 21 patients (17%) during 28 weeks of treatment. EULAR non-responders had antibodies significantly more often than good responders (34% vs 5%; p = 0.032). Patients with antibodies showed less improvement in disease activity (mean (SD) delta DAS28 0.65 (1.35)) than patients without antibodies (mean delta DAS28 1.70 (1.35)) (p = 0.001). Patients with antibodies during follow-up had lower serum adalimumab concentrations at 28 weeks than patients without antibodies (median 1.2 mg/l, range 0.0-5.6 vs median 11.0 mg/l, range 2.0-33.0, respectively; p<0.001). Good responders had higher serum adalimumab concentrations than moderate responders (p = 0.021) and non-responders (p = 0.001). Concomitant methotrexate use was lower in the group with anti-adalimumab antibodies (52%) than in the group without antibodies (84%) (p = 0.003).
Conclusions: Serum antibodies against adalimumab are associated with lower serum adalimumab concentrations and non-response to adalimumab treatment.
Conflict of interest statement
Competing interests: Professor Dijkmans and Professor Tak are members of the advisory board of Abbott and have received honoraria for lectures.
References
-
- Olsen N J, Stein C M. New drugs for rheumatoid arthritis. N Engl J Med 20043502167–2179. - PubMed
-
- Maini R N, Breedveld F C, Kalden J R, Smolen J S, Davis D, Macfarlane J D.et al Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998411552–1563. - PubMed
-
- Hwang W Y, Foote J. Immunogenicity of engineered antibodies. Methods 2005363–10. - PubMed