Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7
- PMID: 17301153
- PMCID: PMC1900170
- DOI: 10.1128/JVI.02498-06
Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7
Abstract
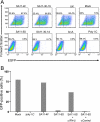
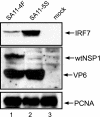
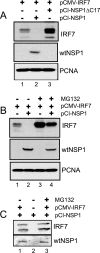
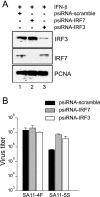
Secretion of interferon (IFN) by virus-infected cells is essential for activating autocrine and paracrine pathways that promote cellular transition to an antiviral state. In most mammalian cells, IFN production is initiated by the activation of constitutively expressed IFN regulatory factor 3, IRF3, which in turn leads to the induction of IRF7, the "master regulator" of IFN type I synthesis (alpha/beta IFN). Previous studies established that rotavirus NSP1 antagonizes IFN signaling by inducing IRF3 degradation. In the present study, we have determined that, in comparison to wild-type rotaviruses, rotaviruses encoding defective NSP1 grow to lower titers in some cell lines and that this poor growth phenotype is due to their failure to suppress IFN expression. Furthermore, we provide evidence that rotaviruses encoding wild-type NSP1 subvert IFN signaling by inducing the degradation of not only IRF3, but also IRF7, with both events occurring through proteasome-dependent processes that proceed with similar efficiencies. The capacity of NSP1 to induce IRF7 degradation may allow rotavirus to move across the gut barrier by enabling the virus to replicate in specialized trafficking cells (dendritic cells and macrophages) that constitutively express IRF7. Along with IRF3 and IRF7, NSP1 was found to induce the degradation of IRF5, a factor that upregulates IFN expression and that is involved in triggering apoptosis during viral infection. Our analysis suggests that NSP1 mediates the degradation of IRF3, IRF5, and IRF7 by recognizing a common element of IRF proteins, thereby allowing NSP1 to act as a broad-spectrum antagonist of IRF function.
Figures



References
-
- Au, W. C., P. A. Moore, D. W. LaFleur, B. Tombal, and P. M. Pitha. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210-29217. - PubMed
-
- Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 22:59-71. - PubMed
-
- Barnes, B. J., M. J. Kellum, K. E. Pinder, J. A. Frisancho, and P. M. Pitha. 2003. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 63:6424-6431. - PubMed
-
- Bowie, A. G., and I. R. Haga. 2005. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 42:859-867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

