Pancortin-2 interacts with WAVE1 and Bcl-xL in a mitochondria-associated protein complex that mediates ischemic neuronal death
- PMID: 17301160
- PMCID: PMC6673736
- DOI: 10.1523/JNEUROSCI.5154-06.2007
Pancortin-2 interacts with WAVE1 and Bcl-xL in a mitochondria-associated protein complex that mediates ischemic neuronal death
Abstract
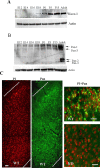
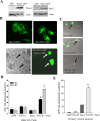
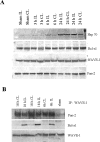
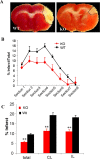
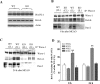
The actin-modulating protein Wiskott-Aldrich syndrome protein verprolin homologous-1 (WAVE1) and a novel CNS-specific protein, pancortin, are highly enriched in adult cerebral cortex, but their functions are unknown. Here we show that WAVE1 and pancortin-2 interact in a novel cell death cascade in adult, but not embryonic, cerebral cortical neurons. Focal ischemic stroke induces the formation of a protein complex that includes pancortin-2, WAVE1, and the anti-apoptotic protein Bcl-xL. The three-protein complex is associated with mitochondria resulting in increased association of Bax with mitochondria, cytochrome c release, and neuronal apoptosis. In pancortin null mice generated using a Cre-loxP system, ischemia-induced WAVE1-Bcl-xL interaction is diminished, and cortical neurons in these mice are protected against ischemic injury. Thus, pancortin-2 is a mediator of ischemia-induced apoptosis of neurons in the adult cerebral cortex and functions in a novel mitochondrial/actin-associated protein complex that sequesters Bcl-xL.
Figures



References
-
- Ando K, Nagano T, Nakamura A, Konno D, Yagi H, Sato M. Expression and characterization of disulfide bond use of oligomerized A2-pancortins: extracellular matrix constituents in the developing brain. Neuroscience. 2005;133:947–957. - PubMed
-
- Banzai Y, Miki H, Yamaguchi H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in neurite extension in PC12 cells and rat hippocampal primary culture cells. J Biol Chem. 2000;275:11987–11992. - PubMed
-
- Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem. 2002;277:49360–49365. - PubMed
-
- Carnegie GK, Scott JD. A-kinase anchoring proteins and neuronal signaling mechanisms. Genes Dev. 2003;17:1557–1568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
