Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception
- PMID: 17301163
- PMCID: PMC6673749
- DOI: 10.1523/JNEUROSCI.3536-06.2007
Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception
Abstract
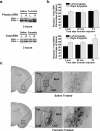
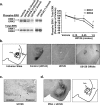
The amygdala has been proposed to serve as a neural center for the modulation of pain perception. Numerous anatomical and behavioral studies demonstrate that exogenous manipulations of the amygdala (i.e., lesions, drug infusions) modulate behavioral responses to acute noxious stimuli; however, little is known about the endogenous molecular changes in the amygdala that contribute to alterations in nociceptive processing during persistent noxious stimuli that resemble pathological pain conditions. In the present study, we demonstrate that endogenous molecular changes in the amygdala play a crucial role in modulating long-lasting peripheral hypersensitivity associated with persistent inflammation and we further identify the extracellular signal-regulated kinase (ERK) as a molecular substrate underlying this behavioral sensitization. Using the formalin test as a mouse model of persistent inflammatory pain, we show that activation of ERK in the amygdala is both necessary for and sufficient to induce long-lasting peripheral hypersensitivity to tactile stimulation. Thus, blockade of inflammation-induced ERK activation in the amygdala significantly reduced long-lasting peripheral hypersensitivity associated with persistent inflammation, and pharmacological activation of ERK in the amygdala induced peripheral hypersensitivity in the absence of inflammation. Importantly, blockade of ERK activation in the amygdala did not affect responses to acute noxious stimuli in the absence of inflammation, indicating that modulation of nociceptive responses by amygdala ERK activation is specific to the persistent inflammatory state. Altogether, our results demonstrate a functional role of the ERK signaling cascade in the amygdala in inflammation-induced peripheral hypersensitivity.
Figures



References
-
- Adamec RE, Blundell J, Burton P. Phosphorylated cyclic AMP response element binding protein expression induced in the periaqueductal gray by predator stress: its relationship to the stress experience, behavior and limbic neural plasticity. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1243–1267. - PubMed
-
- Aggleton JP. The amygdala: a functional analysis. Ed 2. Oxford: Oxford UP; 2000.
-
- Aloisi AM, Porro CA, Cavazzuti M, Baraldi P, Carli G. “Mirror pain” in the formalin test: behavioral and 2-deoxyglucose studies. Pain. 1993;55:267–273. - PubMed
-
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci. 2004;118:15–23. - PubMed
-
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–R76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
