Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition
- PMID: 17301178
- PMCID: PMC6673735
- DOI: 10.1523/JNEUROSCI.5055-06.2007
Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition
Abstract
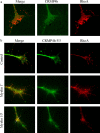
Myelin-associated inhibitors (MAIs) and chondroitin sulfate proteoglycans (CSPGs) contribute to failed regeneration after neuronal injury. MAIs and CSPGs stimulate intracellular signals including the activation of RhoA and Rho kinase to block axonal extension through targeted modifications to the cytoskeleton. RhoA and ROCK are promising targets for therapeutic intervention to promote CNS repair; however, their ubiquitous expression will limit the specificity of drugs targeted to these molecules. We have identified the cytosolic phosphoprotein CRMP4b (collapsin-response mediator protein 4b) as a protein that physically and functionally interacts with RhoA to mediate neurite outgrowth inhibition. Short interfering RNA-mediated knockdown of CRMP4 promotes neurite outgrowth on myelin substrates, indicating a critical role for CRMP4 in neurite outgrowth inhibition. Disruption of CRMP4b-RhoA binding with a competitive inhibitor attenuates neurite outgrowth inhibition on myelin and aggrecan substrates. Stimulation of neuronal growth cones with Nogo leads to colocalization of CRMP4b and RhoA at discrete regions within the actin-rich central and peripheral domains of the growth cone, indicative of a potential function in cytoskeletal rearrangements during neurite outgrowth inhibition. Together, these data indicate that a RhoA-CRMP4b complex forms in response to inhibitory challenges in the growth cone environment and regulates cytoskeletal dynamics at distinct sites necessary for axon outgrowth inhibition. Competitive inhibition of CRMP4b-RhoA binding suggests a novel, highly specific therapeutic avenue for promoting regeneration after CNS injury.
Figures







References
-
- Alabed YZ, Grados-Munro E, Ferraro GB, Hsieh S, Fournier AE. Neuronal responses to myelin are mediated by ROCK. J Neurochem. 2006;96:1616–1625. - PubMed
-
- Arthur WT, Ellerbroek SM, Der CJ, Burridge K, Wennerberg K. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J Biol Chem. 2002;277:42964–42972. - PubMed
-
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22:405–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
