Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit
- PMID: 17301223
- PMCID: PMC1815292
- DOI: 10.1073/pnas.0611532104
Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit
Abstract
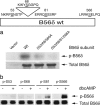
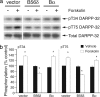
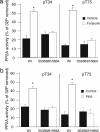
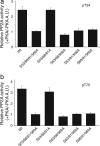
Our previous studies of DARPP-32 in striatal slices have shown that activation of D1 receptors leads to cAMP-dependent dephosphorylation of Thr-75, the Cdk5 site in DARPP-32. In the current study, we have elucidated a mechanism whereby protein phosphatase 2A (PP2A) is activated by a cAMP/PKA-dependent pathway, leading to dephosphorylation of Thr-75. PP2A consists of a catalytic C subunit that associates with the scaffolding A subunit and a variety of B subunits. We have found that the A/C subunits of PP2A, in association with the B56delta (or PPP2R5D) regulatory subunit, is an active DARPP-32 phosphatase. The B56delta subunit expressed in HEK293 cells forms a heterotrimeric assembly that catalyzes PKA-mediated dephosphorylation at Thr-75 in DARPP-32 (also cotransfected into HEK293 cells). The B56delta subunit is phosphorylated by PKA, and this increases the overall activity of PP2A in vitro and in vivo. Among four PKA-phosphorylation sites identified in B56delta in vitro, Ser-566 was found to be critical for the regulation of PP2A activity. Moreover, Ser-566 was phosphorylated by PKA in response to activation of D1 receptors in striatal slices. Based on these studies, we propose that the B56delta/A/C PP2A complex regulates the dephosphorylation of DARPP-32 at Thr-75, thereby helping coordinate the efficacy of dopaminergic neurotransmission in striatal neurons. Moreover, stimulation of protein phosphatase activity by this mechanism may represent an important signaling pathway regulated by cAMP in neurons and other types of cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Greengard P, Allen PB, Nairn AC. Neuron. 1999;23:435–447. - PubMed
-
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. Annu Rev Pharmacol Toxicol. 2004;44:269–296. - PubMed
-
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, et al. Science. 1998;281:838–842. - PubMed
-
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Science. 2003;302:1412–1415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

