Establishing the entatic state in folding metallated Pseudomonas aeruginosa azurin
- PMID: 17301232
- PMCID: PMC1805512
- DOI: 10.1073/pnas.0611149104
Establishing the entatic state in folding metallated Pseudomonas aeruginosa azurin
Abstract
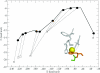
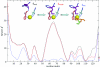
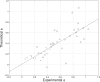
Understanding how the folding of proteins establishes their functional characteristics at the molecular level challenges both theorists and experimentalists. The simplest test beds for confronting this issue are provided by electron transfer proteins. The environment provided by the folded protein to the cofactor tunes the metal's electron transport capabilities as envisioned in the entatic hypothesis. To see how the entatic state is achieved one must study how the folding landscape affects and in turn is affected by the metal. Here, we develop a coarse-grained functional to explicitly model how the coordination of the metal (which results in a so-called entatic or rack-induced state) modifies the folding of the metallated Pseudomonas aeruginosa azurin. Our free-energy functional-based approach directly yields the proper nonlinear extra-thermodynamic free energy relationships for the kinetics of folding the wild type and several point-mutated variants of the metallated protein. The results agree quite well with corresponding laboratory experiments. Moreover, our modified free-energy functional provides a sufficient level of detail to explicitly model how the geometric entatic state of the metal modifies the dynamic folding nucleus of azurin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

