Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes
- PMID: 17301958
- PMCID: PMC2929812
- DOI: 10.1002/jcp.21021
Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes
Abstract
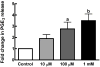
Mechanical loads are required for optimal bone mass. One mechanism whereby mechanical loads are transduced into localized cellular signals is strain-induced fluid flow through lacunae and canaliculi of bone. Gap junctions (GJs) between osteocytes and osteoblasts provides a mechanism whereby flow-induced signals are detected by osteocytes and transduced to osteoblasts. We have demonstrated the importance of GJ and gap junctional intercellular communication (GJIC) in intracellular calcium and prostaglandin E(2) (PGE(2)) increases in response to flow. Unapposed connexons, or hemichannels, are themselves functional and may constitute a novel mechanotransduction mechanism. Using MC3T3-E1 osteoblasts and MLO-Y4 osteocytes, we examined the time course and mechanism of hemichannel activation in response to fluid flow, the composition of the hemichannels, and the role of hemichannels in flow-induced ATP release. We demonstrate that fluid flow activates hemichannels in MLO-Y4, but not MC3T3-E1, through a mechanism involving protein kinase C, which induces ATP and PGE(2) release.
Figures





References
-
- Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone. 2003;33(1):64–70. - PubMed
-
- Bonewald LF. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. J Bone Miner Metab. 1999;17(1):61–65. - PubMed
-
- Brambilla R, Abbracchio MP. Modulation of cyclooxygenase-2 and brain reactive astrogliosis by purinergic P2 receptors. Annals of the New York Academy of Sciences. 2001;939:54–62. - PubMed
-
- Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. Faseb J. 2001;15(1):10–12. - PubMed
-
- Buckley MJ, Banes AJ, Levin LG, Sumpio BE, Sato M, Jordan R, Gilbert J, Link GW, Tran Son Tay R. Osteoblasts increase their rate of division and align in response to cyclic, mechanical tension in vitro. Bone and Mineral. 1988;4(3):225–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

