Tolerance and immunological changes of chemically modified allergen vaccine of Parietaria judaica in accelerated schedules
- PMID: 17302898
- PMCID: PMC1810491
- DOI: 10.1111/j.1365-2249.2006.03309.x
Tolerance and immunological changes of chemically modified allergen vaccine of Parietaria judaica in accelerated schedules
Abstract
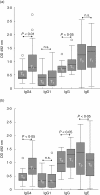
The physicochemical modification of allergen vaccines provides a chance for administering higher doses in a shorter period of time. We sought to assess the safety and immunological changes of using a biologically standardized and modified Parietaria judaica pollen extract in accelerated schedules. Two accelerated schedules were tested in 45 P. judaica-allergic patients: 20 patients reached the maximum dose after two visits using two different concentrations and 25 patients reached the maximum dose after only one visit with two injections of the maximum concentration vial. The tolerance was assessed by recording all side effects related with immunotherapy. Specific antibody levels against native extract and rPar j 2 allergen were evaluated at the beginning and the end of the study. Allergenic potency determined by enzyme allergosorbent test (EAST) inhibition and skin prick test showed that modified P. judaica pollen had a 99.9% less allergenicity than native extract. After 650 doses administered, two clinically irrelevant local reactions (diameter<0 x 5 cm) and no systemic reactions were registered. Significant increases in allergen-specific IgG4 and IgG against P. judaica extract and rPar j 2 and significant decrease of specific IgE against Par j 2 were observed. The modified extract of P. judaica is safe to treat sensitive patients, even at accelerated regimens, and induces significant immunological changes.
Figures

References
-
- The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema. ISAAC Lancet. 1998;351:1225–32. - PubMed
-
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. Allergy. 1998;53:1–42. - PubMed
-
- Bousquet J, Calvayrac P, Guerin B. Immunotherapy with a standardized Dermatphagoides pteronyssinus extract. I. In vivo and in vitro parameters after a short course of treatment. J Allergy Clin Immunol. 1985;76:734–44. - PubMed
-
- Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities from immunotherapy (IT) and skin testing (ST) J Allergy Clin Immunol. 1987;79:660–77. - PubMed
-
- Ferrer M, Burches E, Pelaez A, et al. Double-blind, placebo-controlled study of immunotherpay with Parietaria judaica: clinical efficacy and tolerance. J Invest Allergol Clin Immunol. 2005;15:283–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

