Histone deacetylase inhibitors induce apoptosis in both Type I and Type II endometrial cancer cells
- PMID: 17303224
- PMCID: PMC3273418
- DOI: 10.1016/j.ygyno.2007.01.012
Histone deacetylase inhibitors induce apoptosis in both Type I and Type II endometrial cancer cells
Abstract
Objective: To characterize the molecular pathways involved in apoptosis following administration of histone deacetylase inhibitors to Type I and II endometrial cancer cells.
Methods: Ark2, Ishikawa, and AN3 cell lines representing both Type I and II endometrial cancers were treated with various concentrations of oxamflatin and HDAC inhibitor-1. Cell apoptosis was determined by flow cytometry, nuclear staining, Western blotting, and mitochondrial membrane potential assays.
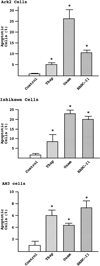
Results: Compared to controls, there was a 95% reduction in the growth of Ark2 cells following administration of histone deacetylase inhibitors and this response was dose-dependent. These agents also caused profound morphologic changes and loss of mitochondrial membrane potentials consistent with the induction of apoptosis. Cleavage of PARP, caspase-9, and caspase-8 was detected, confirming the activation of apoptotic cascades in endometrial carcinoma cells. This effect was present in both serous and endometrioid cell types.
Conclusion: Our results suggest that oxamflatin and HDAC inhibitor-1 have potent cytotoxicity in endometrial cancer cells by inducing cell apoptosis. Histone deacetylase inhibitors are promising agents for the treatment of both Type I and II endometrial carcinoma.
Figures





Similar articles
-
Histone Deacetylase Inhibitors: A Promising Therapeutic Alternative for Endometrial Carcinoma.Dis Markers. 2021 Nov 12;2021:7850688. doi: 10.1155/2021/7850688. eCollection 2021. Dis Markers. 2021. PMID: 34804263 Free PMC article. Review.
-
Histone deacetylase inhibitors and paclitaxel cause synergistic effects on apoptosis and microtubule stabilization in papillary serous endometrial cancer cells.Mol Cancer Ther. 2006 Nov;5(11):2767-76. doi: 10.1158/1535-7163.MCT-06-0209. Mol Cancer Ther. 2006. PMID: 17121923
-
[Effects of trichostatin A and paclitaxel on apoptosis and mitochondrial membrane potential of human endometrial carcinoma Ark2 cells].Ai Zheng. 2008 Aug;27(8):816-21. Ai Zheng. 2008. PMID: 18710614 Chinese.
-
Cytostatic and apoptotic effects of DNMT and HDAC inhibitors in endometrial cancer cells.Curr Pharm Des. 2014;20(11):1881-7. doi: 10.2174/13816128113199990527. Curr Pharm Des. 2014. PMID: 23888960
-
Human endometrial and ovarian cancer cells: histone deacetylase inhibitors exhibit antiproliferative activity, potently induce cell cycle arrest, and stimulate apoptosis.Curr Med Chem. 2007;14(24):2548-53. doi: 10.2174/092986707782023299. Curr Med Chem. 2007. PMID: 17979707 Review.
Cited by
-
Histone Deacetylase Inhibitors: A Promising Therapeutic Alternative for Endometrial Carcinoma.Dis Markers. 2021 Nov 12;2021:7850688. doi: 10.1155/2021/7850688. eCollection 2021. Dis Markers. 2021. PMID: 34804263 Free PMC article. Review.
-
Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas.Neoplasia. 2008 Sep;10(9):1021-7. doi: 10.1593/neo.08474. Neoplasia. 2008. PMID: 18714364 Free PMC article.
-
Reversible inhibition of lysine specific demethylase 1 is a novel anti-tumor strategy for poorly differentiated endometrial carcinoma.BMC Cancer. 2014 Oct 9;14:752. doi: 10.1186/1471-2407-14-752. BMC Cancer. 2014. PMID: 25300887 Free PMC article.
-
Combination of palladium nanoparticles and tubastatin-A potentiates apoptosis in human breast cancer cells: a novel therapeutic approach for cancer.Int J Nanomedicine. 2017 Sep 5;12:6503-6520. doi: 10.2147/IJN.S136142. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28919751 Free PMC article.
-
Histone Deacetylase 3 Governs β-Estradiol-ERα-Involved Endometrial Tumorigenesis via Inhibition of STING Transcription.Cancers (Basel). 2022 Sep 28;14(19):4718. doi: 10.3390/cancers14194718. Cancers (Basel). 2022. PMID: 36230643 Free PMC article.
References
-
- Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004 Mar;444(3):213–223. - PubMed
-
- Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000 Jun 7;92(11):924–930. - PubMed
-
- MacDonald ND, Salvesen HB, Ryan A, Iversen OE, Akslen LA, Jacobs IJ. Frequency and prognostic impact of microsatellite instability in a large population-based study of endometrial carcinomas. Cancer Res. 2000 Mar 15;60(6):1750–1752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

