Towards hyperpolarized (13)C-succinate imaging of brain cancer
- PMID: 17303454
- PMCID: PMC2657725
- DOI: 10.1016/j.jmr.2007.01.017
Towards hyperpolarized (13)C-succinate imaging of brain cancer
Abstract
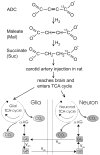
We describe a novel (13)C enriched precursor molecule, sodium 1-(13)C acetylenedicarboxylate, which after hydrogenation by PASADENA (Parahydrogen and Synthesis Allows Dramatically Enhanced Nuclear Alignment) under controlled experimental conditions, becomes hyperpolarized (13)C sodium succinate. Fast in vivo 3D FIESTA MR imaging demonstrated that, following carotid arterial injection, the hyperpolarized (13)C-succinate appeared in the head and cerebral circulation of normal and tumor-bearing rats. At this time, no in vivo hyperpolarized signal has been localized to normal brain or brain tumor. On the other hand, ex vivo samples of brain harvested from rats bearing a 9L brain tumor, 1 h or more following in vivo carotid injection of hyperpolarized (13)C sodium succinate, contained significant concentrations of the injected substrate, (13)C sodium succinate, together with (13)C maleate and succinate metabolites 1-(13)C-glutamate, 5-(13)C-glutamate, 1-(13)C-glutamine and 5-(13)C-glutamine. The (13)C substrates and products were below the limits of NMR detection in ex vivo samples of normal brain consistent with an intact blood-brain barrier. These ex vivo results indicate that hyperpolarized (13)C sodium succinate may become a useful tool for rapid in vivo identification of brain tumors, providing novel biomarkers in (13)C MR spectral-spatial images.
Figures



References
-
- Edelman R, Hesselink J, Zlatkin M. Clinical Magnetic Resonance Imaging. W.B. Saunders and Co.; Philadelphia: 1996.
-
- Magnetic Resonance at Solid State Physics and Magnetic Resonance Service in Saclay. Bulletin D Informations Scientifiques Et Techniques. 1969:3.
-
- Modern Techniques in Neuroscience Research. Springer–Verlag; 1999.
-
- Cerdan S. NMR in Biomedicine. Wiley, NY; 2003.
-
- Ross B, Lin A, Harris K, Bhattacharya P, Schweinsburg B. Clinical experience with C-13 MRS in vivo. NMR Biomed. 2003;16:358–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

