Thylakoid membrane perforations and connectivity enable intracellular traffic in cyanobacteria
- PMID: 17304210
- PMCID: PMC1817639
- DOI: 10.1038/sj.emboj.7601594
Thylakoid membrane perforations and connectivity enable intracellular traffic in cyanobacteria
Abstract
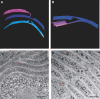
Cyanobacteria, the progenitors of plant and algal chloroplasts, enabled aerobic life on earth by introducing oxygenic photosynthesis. In most cyanobacteria, the photosynthetic membranes are arranged in multiple, seemingly disconnected, concentric shells. In such an arrangement, it is unclear how intracellular trafficking proceeds and how different layers of the photosynthetic membranes communicate with each other to maintain photosynthetic homeostasis. Using electron microscope tomography, we show that the photosynthetic membranes of two distantly related cyanobacterial species contain multiple perforations. These perforations, which are filled with particles of different sizes including ribosomes, glycogen granules and lipid bodies, allow for traffic throughout the cell. In addition, different layers of the photosynthetic membranes are joined together by internal bridges formed by branching and fusion of the membranes. The result is a highly connected network, similar to that of higher-plant chloroplasts, allowing water-soluble and lipid-soluble molecules to diffuse through the entire membrane network. Notably, we observed intracellular membrane-bounded vesicles, which were frequently fused to the photosynthetic membranes and may play a role in transport to these membranes.
Figures




Similar articles
-
Three-dimensional ultrastructure of a unicellular cyanobacterium.J Cell Biol. 1983 Sep;97(3):713-22. doi: 10.1083/jcb.97.3.713. J Cell Biol. 1983. PMID: 6411738 Free PMC article.
-
Unique thylakoid membrane architecture of a unicellular N2-fixing cyanobacterium revealed by electron tomography.Plant Physiol. 2011 Apr;155(4):1656-66. doi: 10.1104/pp.110.165332. Epub 2010 Dec 20. Plant Physiol. 2011. PMID: 21173021 Free PMC article.
-
Co-existence of photosynthetic and respiratory activities in cyanobacterial thylakoid membranes.Biochim Biophys Acta. 2014 Apr;1837(4):503-11. doi: 10.1016/j.bbabio.2013.11.017. Epub 2013 Dec 6. Biochim Biophys Acta. 2014. PMID: 24316145 Review.
-
The plasma membrane of the cyanobacterium Gloeobacter violaceus contains segregated bioenergetic domains.Plant Cell. 2011 Jun;23(6):2379-90. doi: 10.1105/tpc.111.085779. Epub 2011 Jun 3. Plant Cell. 2011. PMID: 21642550 Free PMC article.
-
Thylakoid Development and Galactolipid Synthesis in Cyanobacteria.Subcell Biochem. 2016;86:85-101. doi: 10.1007/978-3-319-25979-6_4. Subcell Biochem. 2016. PMID: 27023232 Review.
Cited by
-
Effect of Different Broad Waveband Lights on Membrane Lipids of a Cyanobacterium, Synechococcus sp., as Determined by UPLC-QToF-MS and Vibrational Spectroscopy.Biology (Basel). 2016 May 23;5(2):22. doi: 10.3390/biology5020022. Biology (Basel). 2016. PMID: 27223306 Free PMC article.
-
Deletion of Synechocystis sp. PCC 6803 leader peptidase LepB1 affects photosynthetic complexes and respiration.Mol Cell Proteomics. 2013 May;12(5):1192-203. doi: 10.1074/mcp.M112.022145. Epub 2013 Jan 28. Mol Cell Proteomics. 2013. PMID: 23358502 Free PMC article.
-
The vesicle-inducing protein 1 from Synechocystis sp. PCC 6803 organizes into diverse higher-ordered ring structures.Mol Biol Cell. 2009 Nov;20(21):4620-8. doi: 10.1091/mbc.e09-04-0319. Epub 2009 Sep 23. Mol Biol Cell. 2009. PMID: 19776353 Free PMC article.
-
Glycogen synthase isoforms in Synechocystis sp. PCC6803: identification of different roles to produce glycogen by targeted mutagenesis.PLoS One. 2014 Mar 17;9(3):e91524. doi: 10.1371/journal.pone.0091524. eCollection 2014. PLoS One. 2014. PMID: 24637565 Free PMC article.
-
A note on three-dimensional models of higher-plant thylakoid networks.Plant Cell. 2008 Oct;20(10):2546-9; author reply 2549-51. doi: 10.1105/tpc.108.062299. Epub 2008 Oct 24. Plant Cell. 2008. PMID: 18952775 Free PMC article. No abstract available.
References
-
- Adir N, Zer H, Shochat S, Ohad I (2003) Photoinhibition—a historical perspective. Photosynth Res 76: 343–370 - PubMed
-
- Balint I, Bhattacharya J, Perelman A, Schatz D, Moskovitz Y, Keren N, Schwarz R (2006) Inactivation of the extrinsic subunit of photosystem II, PsbU, in Synechococcus PCC 7942 results in elevated resistance to oxidative stress. FEBS Lett 580: 2117–2122 - PubMed
-
- Barber J, Andersson B (1992) Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci 17: 61–66 - PubMed
-
- Brangeon J, Mustardy L (1979) Ontogenetic assembly of intra-chloroplastic lamellae viewed in 3-dimension. Biol Cell 36: 71–80
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

