Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation
- PMID: 17304211
- PMCID: PMC1817635
- DOI: 10.1038/sj.emboj.7601587
Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation
Abstract
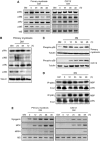
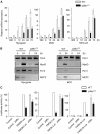
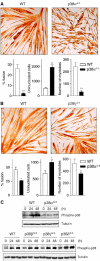
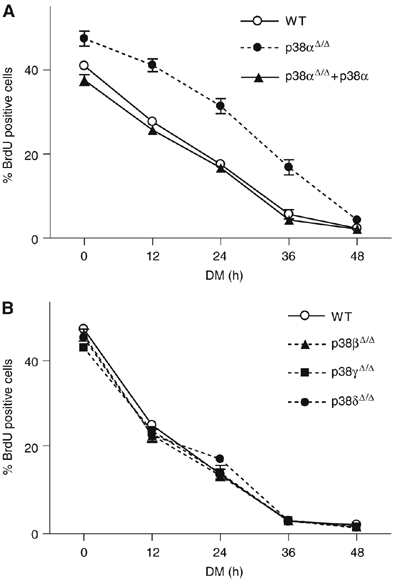
The p38 mitogen-activated protein kinase (MAPK) pathway plays a critical role in skeletal muscle differentiation. However, the relative contribution of the four p38 MAPKs (p38alpha, p38beta, p38gamma and p38delta) to this process is unknown. Here we show that myoblasts lacking p38alpha, but not those lacking p38beta or p38delta, are unable to differentiate and form multinucleated myotubes, whereas p38gamma-deficient myoblasts exhibit an attenuated fusion capacity. The defective myogenesis in the absence of p38alpha is caused by delayed cell-cycle exit and continuous proliferation in differentiation-promoting conditions. Indeed, activation of JNK/cJun was enhanced in p38alpha-deficient myoblasts leading to increased cyclin D1 transcription, whereas inhibition of JNK activity rescued the proliferation phenotype. Thus, p38alpha controls myogenesis by antagonizing the activation of the JNK proliferation-promoting pathway, before its direct effect on muscle differentiation-specific gene transcription. More importantly, in agreement with the defective myogenesis of cultured p38alpha(Delta/Delta) myoblasts, neonatal muscle deficient in p38alpha shows cellular hyperproliferation and delayed maturation. This study provides novel evidence of a fundamental role of p38alpha in muscle formation in vitro and in vivo.
Figures



Similar articles
-
Genetic deficiency of p38alpha reveals its critical role in myoblast cell cycle exit: the p38alpha-JNK connection.Cell Cycle. 2007 Jun 1;6(11):1298-303. doi: 10.4161/cc.6.11.4315. Epub 2007 Jun 20. Cell Cycle. 2007. PMID: 17534150 Review.
-
Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages.J Immunol. 1999 Apr 1;162(7):4246-52. J Immunol. 1999. PMID: 10201954
-
Efficient adult skeletal muscle regeneration in mice deficient in p38beta, p38gamma and p38delta MAP kinases.Cell Cycle. 2008 Jul 15;7(14):2208-14. doi: 10.4161/cc.7.14.6273. Epub 2008 May 12. Cell Cycle. 2008. PMID: 18641461
-
Convergence of Igf2 expression and adhesion signalling via RhoA and p38 MAPK enhances myogenic differentiation.J Cell Sci. 2006 Dec 1;119(Pt 23):4828-40. doi: 10.1242/jcs.03278. Epub 2006 Nov 14. J Cell Sci. 2006. PMID: 17105766
-
Novel strategies for inhibition of the p38 MAPK pathway.Trends Pharmacol Sci. 2007 Jun;28(6):286-95. doi: 10.1016/j.tips.2007.04.008. Epub 2007 May 7. Trends Pharmacol Sci. 2007. PMID: 17482683 Review.
Cited by
-
Regulation of Muscle Stem Cell Functions: A Focus on the p38 MAPK Signaling Pathway.Front Cell Dev Biol. 2016 Aug 30;4:91. doi: 10.3389/fcell.2016.00091. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27626031 Free PMC article. Review.
-
Hsp70 Interacts with Mitogen-Activated Protein Kinase (MAPK)-Activated Protein Kinase 2 To Regulate p38MAPK Stability and Myoblast Differentiation during Skeletal Muscle Regeneration.Mol Cell Biol. 2018 Nov 28;38(24):e00211-18. doi: 10.1128/MCB.00211-18. Print 2018 Dec 15. Mol Cell Biol. 2018. PMID: 30275345 Free PMC article.
-
Signal integration by JNK and p38 MAPK pathways in cancer development.Nat Rev Cancer. 2009 Aug;9(8):537-49. doi: 10.1038/nrc2694. Nat Rev Cancer. 2009. PMID: 19629069 Review.
-
Cdo binds Abl to promote p38alpha/beta mitogen-activated protein kinase activity and myogenic differentiation.Mol Cell Biol. 2009 Aug;29(15):4130-43. doi: 10.1128/MCB.00199-09. Epub 2009 May 26. Mol Cell Biol. 2009. PMID: 19470755 Free PMC article.
-
Geriatric muscle stem cells switch reversible quiescence into senescence.Nature. 2014 Feb 20;506(7488):316-21. doi: 10.1038/nature13013. Epub 2014 Feb 12. Nature. 2014. PMID: 24522534
References
-
- Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR (2000) Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell 6: 109–116 - PubMed
-
- Angel P, Hattori K, Smeal T, Karin M (1988) The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell 55: 875–885 - PubMed
-
- Bennett AM, Tonks NK (1997) Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278: 1288–1291 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

