Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines
- PMID: 17304353
- PMCID: PMC1794116
- DOI: 10.1172/JCI28180
Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines
Abstract
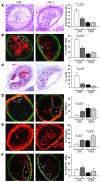
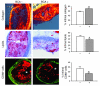
We previously found that low shear stress (LSS) induces atherosclerotic plaques in mice with increased lipid and matrix metalloproteinase content and decreased vascular smooth muscle and collagen content. Here, we evaluated the role of chemokines in this process, using an extravascular device inducing regions of LSS, high shear stress, and oscillatory shear stress (OSS) in the carotid artery. One week of shear stress alterations induced expression of IFN-gamma-inducible protein-10 (IP-10) exclusively in the LSS region, whereas monocyte chemoattractant protein-1 (MCP-1) and the mouse homolog of growth-regulated oncogene alpha (GRO-alpha) were equally upregulated in both LSS and OSS regions. After 3 weeks, GRO-alpha and IP-10 were specifically upregulated in LSS regions. After 9 weeks, lesions with thinner fibrous caps and larger necrotic cores were found in the LSS region compared with the OSS region. Equal levels of MCP-1 expression were observed in both regions, while expression of fractalkine was found in the LSS region only. Blockage of fractalkine inhibited plaque growth and resulted in striking differences in plaque composition in the LSS region. We conclude that LSS or OSS triggers expression of chemokines involved in atherogenesis. Fractalkine upregulation is critically important for the composition of LSS-induced atherosclerotic lesions.
Figures

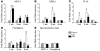
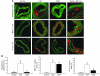
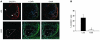
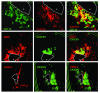
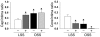

References
-
- Cheng C. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. - PubMed
-
- Williams H., Johnson J.L., Carson K.G., Jackson C.L. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:788–792. - PubMed
-
- Pedersen E.M., et al. Distribution of early atherosclerotic lesions in the human abdominal aorta correlates with wall shear stresses measured in vivo. Eur. J. Vasc. Endovasc. Surg. 1999;18:328–333. - PubMed
-
- Wentzel J.J., et al. Shear-stress and wall-stress regulation of vascular remodeling after balloon angioplasty: effect of matrix metalloproteinase inhibition. Circulation. 2001;104:91–96. - PubMed
-
- Buchanan J.R., Jr., Kleinstreuer C., Truskey G.A., Lei M. Relation between non-uniform hemodynamics and sites of altered permeability and lesion growth at the rabbit aorto-celiac junction. Atherosclerosis. 1999;143:27–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

