The role of myelin in Theiler's virus persistence in the central nervous system
- PMID: 17305428
- PMCID: PMC1797621
- DOI: 10.1371/journal.ppat.0030023
The role of myelin in Theiler's virus persistence in the central nervous system
Abstract
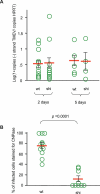
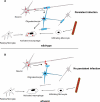
Theiler's virus, a picornavirus, persists for life in the central nervous system of mouse and causes a demyelinating disease that is a model for multiple sclerosis. The virus infects neurons first but persists in white matter glial cells, mainly oligodendrocytes and macrophages. The mechanism, by which the virus traffics from neurons to glial cells, and the respective roles of oligodendrocytes and macrophages in persistence are poorly understood. We took advantage of our previous finding that the shiverer mouse, a mutant with a deletion in the myelin basic protein gene (Mbp), is resistant to persistent infection to examine the role of myelin in persistence. Using immune chimeras, we show that resistance is not mediated by immune responses or by an efficient recruitment of inflammatory cells into the central nervous system. With both in vivo and in vitro experiments, we show that the mutation does not impair the permissiveness of neurons, oligodendrocytes, and macrophages to the virus. We demonstrate that viral antigens are present in cytoplasmic channels of myelin during persistent infection of wild-type mice. Using the optic nerve as a model, we show that the virus traffics from the axons of retinal ganglion cells to the cytoplasmic channels of myelin, and that this traffic is impaired by the shiverer mutation. These results uncover an unsuspected axon to myelin traffic of Theiler's virus and the essential role played by the infection of myelin/oligodendrocyte in persistence.
Conflict of interest statement
Figures


Similar articles
-
Theiler's virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes.Virus Res. 2005 Aug;111(2):214-23. doi: 10.1016/j.virusres.2005.04.010. Virus Res. 2005. PMID: 15893838 Review.
-
The genetics of the persistent infection and demyelinating disease caused by Theiler's virus.Annu Rev Microbiol. 2005;59:279-98. doi: 10.1146/annurev.micro.59.030804.121242. Annu Rev Microbiol. 2005. PMID: 16153171 Review.
-
The shiverer mutation affects the persistence of Theiler's virus in the central nervous system.J Virol. 1997 Jul;71(7):5025-30. doi: 10.1128/JVI.71.7.5025-5030.1997. J Virol. 1997. PMID: 9188567 Free PMC article.
-
Genetics of susceptibility to Theiler's virus infection.Bioessays. 1998 Aug;20(8):627-33. doi: 10.1002/(SICI)1521-1878(199808)20:8<627::AID-BIES5>3.0.CO;2-F. Bioessays. 1998. PMID: 9780837 Review.
-
[Infection of the mouse by Theiler's virus; a model for the study of demyelinating diseases of the central nervous system].Bull Acad Natl Med. 1996 Feb;180(2):305-15; discussion 315-6. Bull Acad Natl Med. 1996. PMID: 8705377 Review. French.
Cited by
-
Microglia in antiviral immunity of the brain and spinal cord.Semin Immunol. 2022 Mar;60:101650. doi: 10.1016/j.smim.2022.101650. Epub 2022 Sep 10. Semin Immunol. 2022. PMID: 36099864 Free PMC article. Review.
-
Inflammation's impact on the interaction between oligodendrocytes and axons.Discov Immunol. 2025 Apr 29;4(1):kyaf008. doi: 10.1093/discim/kyaf008. eCollection 2025. Discov Immunol. 2025. PMID: 40636264 Free PMC article. Review.
-
Axonal spread of neuroinvasive viral infections.Trends Microbiol. 2015 May;23(5):283-8. doi: 10.1016/j.tim.2015.01.002. Epub 2015 Jan 29. Trends Microbiol. 2015. PMID: 25639651 Free PMC article. Review.
-
Identification of a novel neuropathogenic Theiler's murine encephalomyelitis virus.J Virol. 2011 Jul;85(14):6893-905. doi: 10.1128/JVI.00274-11. Epub 2011 May 4. J Virol. 2011. PMID: 21543488 Free PMC article.
-
Axon-myelin interactions during a viral infection of the central nervous system.PLoS Pathog. 2009 Sep;5(9):e1000519. doi: 10.1371/journal.ppat.1000519. Epub 2009 Sep 25. PLoS Pathog. 2009. PMID: 19779566 Free PMC article. No abstract available.
References
-
- Brahic M, Bureau JF, Michiels T. The genetics of the persistent infection and demyelinating disease caused by Theiler's virus. Annu Rev Microbiol. 2005;59:279–298. - PubMed
-
- Aubert C, Chamorro M, Brahic M. Identification of Theiler's virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. - PubMed
-
- Rodriguez M, Leibowitz JL, Lampert PW. Persistent infection of oligodendrocytes in Theiler's virus induced encephalomyelitis. Ann Neurol. 1983;13:426–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

