Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18
- PMID: 17305430
- PMCID: PMC1797815
- DOI: 10.1371/journal.pgen.0030023
Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18
Abstract
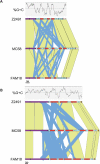
The bacterium Neisseria meningitidis is commonly found harmlessly colonising the mucosal surfaces of the human nasopharynx. Occasionally strains can invade host tissues causing septicaemia and meningitis, making the bacterium a major cause of morbidity and mortality in both the developed and developing world. The species is known to be diverse in many ways, as a product of its natural transformability and of a range of recombination and mutation-based systems. Previous work on pathogenic Neisseria has identified several mechanisms for the generation of diversity of surface structures, including phase variation based on slippage-like mechanisms and sequence conversion of expressed genes using information from silent loci. Comparison of the genome sequences of two N. meningitidis strains, serogroup B MC58 and serogroup A Z2491, suggested further mechanisms of variation, including C-terminal exchange in specific genes and enhanced localised recombination and variation related to repeat arrays. We have sequenced the genome of N. meningitidis strain FAM18, a representative of the ST-11/ET-37 complex, providing the first genome sequence for the disease-causing serogroup C meningococci; it has 1,976 predicted genes, of which 60 do not have orthologues in the previously sequenced serogroup A or B strains. Through genome comparison with Z2491 and MC58 we have further characterised specific mechanisms of genetic variation in N. meningitidis, describing specialised loci for generation of cell surface protein variants and measuring the association between noncoding repeat arrays and sequence variation in flanking genes. Here we provide a detailed view of novel genetic diversification mechanisms in N. meningitidis. Our analysis provides evidence for the hypothesis that the noncoding repeat arrays in neisserial genomes (neisserial intergenic mosaic elements) provide a crucial mechanism for the generation of surface antigen variants. Such variation will have an impact on the interaction with the host tissues, and understanding these mechanisms is important to aid our understanding of the intimate and complex relationship between the human nasopharynx and the meningococcus.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures



Similar articles
-
Repeat-associated phase variable genes in the complete genome sequence of Neisseria meningitidis strain MC58.Mol Microbiol. 2000 Jul;37(1):207-15. doi: 10.1046/j.1365-2958.2000.02000.x. Mol Microbiol. 2000. PMID: 10931317
-
Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491.Nature. 2000 Mar 30;404(6777):502-6. doi: 10.1038/35006655. Nature. 2000. PMID: 10761919
-
Genetic characterization of pilin glycosylation and phase variation in Neisseria meningitidis.Mol Microbiol. 2003 Aug;49(3):833-47. doi: 10.1046/j.1365-2958.2003.03602.x. Mol Microbiol. 2003. PMID: 12864863
-
Genetic shifts of Neisseria meningitidis serogroup B antigens and the quest for a broadly cross-protective vaccine.Expert Rev Vaccines. 2010 Oct;9(10):1203-17. doi: 10.1586/erv.10.116. Expert Rev Vaccines. 2010. PMID: 20923270 Review.
-
Microevolution and Its Impact on Hypervirulence, Antimicrobial Resistance, and Vaccine Escape in Neisseria meningitidis.Microorganisms. 2023 Dec 18;11(12):3005. doi: 10.3390/microorganisms11123005. Microorganisms. 2023. PMID: 38138149 Free PMC article. Review.
Cited by
-
Meningococcal Two-Partner Secretion Systems and Their Association with Outcome in Patients with Meningitis.Infect Immun. 2016 Aug 19;84(9):2534-40. doi: 10.1128/IAI.00160-16. Print 2016 Sep. Infect Immun. 2016. PMID: 27324486 Free PMC article.
-
A novel interpretation of structural dot plots of genomes derived from the analysis of two strains of Neisseria meningitidis.Genomics Proteomics Bioinformatics. 2010 Sep;8(3):159-69. doi: 10.1016/S1672-0229(10)60018-6. Genomics Proteomics Bioinformatics. 2010. PMID: 20970744 Free PMC article.
-
Lipoproteins of bacterial pathogens.Infect Immun. 2011 Feb;79(2):548-61. doi: 10.1128/IAI.00682-10. Epub 2010 Oct 25. Infect Immun. 2011. PMID: 20974828 Free PMC article. Review.
-
The comparative population genetics of Neisseria meningitidis and Neisseria gonorrhoeae.PeerJ. 2019 Jun 27;7:e7216. doi: 10.7717/peerj.7216. eCollection 2019. PeerJ. 2019. PMID: 31293838 Free PMC article.
-
The transposon-like Correia elements encode numerous strong promoters and provide a potential new mechanism for phase variation in the meningococcus.PLoS Genet. 2011 Jan 20;7(1):e1001277. doi: 10.1371/journal.pgen.1001277. PLoS Genet. 2011. PMID: 21283790 Free PMC article.
References
-
- Stephens DS, Hoffman LH, McGee ZA. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: Attachment and entry into columnar epithelial cells. J Infect Dis. 1983;148:369–376. - PubMed
-
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–1388. - PubMed
-
- Tzeng YL, Stephens DS. Epidemiology and pathogenesis of Neisseria meningitidis . Microbes Infect. 2000;2:687–700. - PubMed
-
- Snyder LA, Davies JK, Ryan CS, Saunders NJ. Comparative overview of the genomic and genetic differences between the pathogenic Neisseria strains and species. Plasmid. 2005;54:191–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

