Phenotypic plasticity in Drosophila pigmentation caused by temperature sensitivity of a chromatin regulator network
- PMID: 17305433
- PMCID: PMC1797818
- DOI: 10.1371/journal.pgen.0030030
Phenotypic plasticity in Drosophila pigmentation caused by temperature sensitivity of a chromatin regulator network
Abstract
Phenotypic plasticity is the ability of a genotype to produce contrasting phenotypes in different environments. Although many examples have been described, the responsible mechanisms are poorly understood. In particular, it is not clear how phenotypic plasticity is related to buffering, the maintenance of a constant phenotype against genetic or environmental variation. We investigate here the genetic basis of a particularly well described plastic phenotype: the abdominal pigmentation in female Drosophila melanogaster. Cold temperature induces a dark pigmentation, in particular in posterior segments, while higher temperature has the opposite effect. We show that the homeotic gene Abdominal-B (Abd-B) has a major role in the plasticity of pigmentation in the abdomen. Abd-B plays opposite roles on melanin production through the regulation of several pigmentation enzymes. This makes the control of pigmentation very unstable in the posterior abdomen, and we show that the relative spatio-temporal expression of limiting pigmentation enzymes in this region of the body is thermosensitive. Temperature acts on melanin production by modulating a chromatin regulator network, interacting genetically with the transcription factor bric-à-brac (bab), a target of Abd-B and Hsp83, encoding the chaperone Hsp90. Genetic disruption of this chromatin regulator network increases the effect of temperature and the instability of the pigmentation pattern in the posterior abdomen. Colocalizations on polytene chromosomes suggest that BAB and these chromatin regulators cooperate in the regulation of many targets, including several pigmentation enzymes. We show that they are also involved in sex comb development in males and that genetic destabilization of this network is also strongly modulated by temperature for this phenotype. Thus, we propose that phenotypic plasticity of pigmentation is a side effect reflecting a global impact of temperature on epigenetic mechanisms. Furthermore, the thermosensitivity of this network may be related to the high evolvability of several secondary sexual characters in the genus Drosophila.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
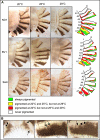
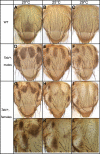



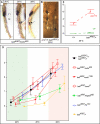
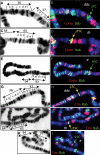
Similar articles
-
Phenotypic Plasticity through Transcriptional Regulation of the Evolutionary Hotspot Gene tan in Drosophila melanogaster.PLoS Genet. 2016 Aug 10;12(8):e1006218. doi: 10.1371/journal.pgen.1006218. eCollection 2016 Aug. PLoS Genet. 2016. PMID: 27508387 Free PMC article.
-
bric à brac (bab), a central player in the gene regulatory network that mediates thermal plasticity of pigmentation in Drosophila melanogaster.PLoS Genet. 2018 Aug 1;14(8):e1007573. doi: 10.1371/journal.pgen.1007573. eCollection 2018 Aug. PLoS Genet. 2018. PMID: 30067846 Free PMC article.
-
Pigmentation and mate choice in Drosophila.Nature. 2002 Sep 26;419(6905):360; discussion 360. doi: 10.1038/419360a. Nature. 2002. PMID: 12353025
-
[Phenotypic plasticity in insects].Biol Aujourdhui. 2020;214(1-2):33-44. doi: 10.1051/jbio/2020005. Epub 2020 Aug 10. Biol Aujourdhui. 2020. PMID: 32773028 Review. French.
-
Pigmentation and behavior: potential association through pleiotropic genes in Drosophila.Genes Genet Syst. 2013;88(3):165-74. doi: 10.1266/ggs.88.165. Genes Genet Syst. 2013. PMID: 24025245 Review.
Cited by
-
A statistical model for testing the pleiotropic control of phenotypic plasticity for a count trait.Genetics. 2008 May;179(1):627-36. doi: 10.1534/genetics.107.081794. Genetics. 2008. PMID: 18493077 Free PMC article.
-
The microRNA miR-8 is a positive regulator of pigmentation and eclosion in Drosophila.Dev Dyn. 2012 Jan;241(1):161-8. doi: 10.1002/dvdy.23705. Dev Dyn. 2012. PMID: 22174085 Free PMC article.
-
Seasonal morphotypes of Drosophila suzukii differ in key life-history traits during and after a prolonged period of cold exposure.Ecol Evol. 2020 Aug 11;10(17):9085-9099. doi: 10.1002/ece3.6517. eCollection 2020 Sep. Ecol Evol. 2020. PMID: 32953048 Free PMC article.
-
The genetic control of aposematic black pigmentation in hemimetabolous insects: insights from Oncopeltus fasciatus.Evol Dev. 2014 Sep;16(5):270-7. doi: 10.1111/ede.12090. Epub 2014 Aug 14. Evol Dev. 2014. PMID: 25124093 Free PMC article.
-
Phenotypic plasticity of abdomen pigmentation in two geographic populations of Drosophila melanogaster: male-female comparison and sexual dimorphism.Genetica. 2009 Apr;135(3):403-13. doi: 10.1007/s10709-008-9286-2. Epub 2008 Jun 22. Genetica. 2009. PMID: 18568431
References
-
- Via S, Gomulkievicz R, de Jong G, Scheiner SM, Schlichting CD, et al. Adaptive phenotypic plasticity: Consensus and controversy. Trend Ecol Evol. 1995;10:212–217. - PubMed
-
- West-Eberhard MJ. Developmental plasticity: An evolution. New York: Oxford University Press; 2003. 794
-
- de Jong G. Evolution of phenotypic plasticity: Patterns of plasticity and the emergence of ecotypes. New Phytol. 2005;166:101–117. - PubMed
-
- Schlichting CD, Smith H. Phenotypic plasticity: Linking molecular mechanisms with evolutionary outcomes. Evol Ecol. 2002;16:189–201.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

