Rat salivary gland ligation causes reversible secretory hypofunction
- PMID: 17305704
- PMCID: PMC1859985
- DOI: 10.1111/j.1365-201X.2006.01662.x
Rat salivary gland ligation causes reversible secretory hypofunction
Abstract
Aim: To determine the influence of inflammation on salivary secretion. Secretion by salivary glands involves interactions between nerves, blood vessels and salivary cells. The present study investigated the effects of inflammation on rat submandibular gland function following acute ductal obstruction.
Methods: Under recovery anaesthesia a metal clip was placed on the main duct of the submandibular gland. After 24 h salivary secretion was evoked by nerve and methacholine stimulation. For recovery experiments the clip was removed after 24 h and the animal left to recover for 3 days when salivary function was again assessed.
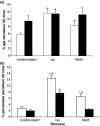
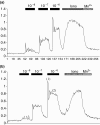
Results: By 24 h of obstruction an inflammatory infiltrate had developed within the obstructed gland and stimulated salivary flows were just 20% of the normal secretion, whilst protein secretion and ion reabsorption were also severely impaired. If ductal obstruction was removed after 24 h the salivary function returned to normal after 3 days of recovery. In vitro analysis of cells from 24-h ligated glands revealed normal changes in intracellular calcium (the main secondary messenger involved in fluid secretion) in response to methacholine stimulation. Protein secretion from isolated cells indicated some changes in particular to methacholine-induced protein secretion although a significant protein secretion was still seen in response to isoprenaline - the main stimulus for protein secretion.
Conclusion: This report demonstrates reversible salivary inhibition associated with an inflammatory infiltrate within the salivary gland.
Figures



References
-
- Adesanya MR, Redman RS, Baum BJ, OConnell BC. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum Gene Ther. 1996;7:1085–1093. - PubMed
-
- Amerongen AVN, Veerman ECI. Saliva – the defender of the oral cavity. Oral Dis. 2002;8:12–22. - PubMed
-
- Anderson LC. Peroxidase release from rat submandibular salivary acinar cells in vitro. Arch Oral Biol. 1986;31:501–502. - PubMed
-
- Anderson LC, Garrett JR. Neural regulation of blood flow in the rat submandibular gland. Eur J Morphol. 1998;36:213–218. - PubMed
-
- Baum BJ, Kuyatt BL. Protein production and release by dispersed rat submandibular cells in vitro after adrenergic stimulation. Life Sci. 1981;29:1143–1149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

