Mechanisms of action of glucagon-like peptide 1 in the pancreas
- PMID: 17306374
- PMCID: PMC1934514
- DOI: 10.1016/j.pharmthera.2006.11.007
Mechanisms of action of glucagon-like peptide 1 in the pancreas
Abstract
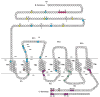
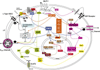
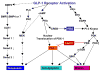
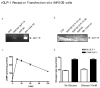
Glucagon-like peptide 1 (GLP-1) is a hormone that is encoded in the proglucagon gene. It is mainly produced in enteroendocrine L cells of the gut and is secreted into the blood stream when food containing fat, protein hydrolysate, and/or glucose enters the duodenum. Its particular effects on insulin and glucagon secretion have generated a flurry of research activity over the past 20 years culminating in a naturally occurring GLP-1 receptor (GLP-1R) agonist, exendin 4 (Ex-4), now being used to treat type 2 diabetes mellitus (T2DM). GLP-1 engages a specific guanine nucleotide-binding protein (G-protein) coupled receptor (GPCR) that is present in tissues other than the pancreas (brain, kidney, lung, heart, and major blood vessels). The most widely studied cell activated by GLP-1 is the insulin-secreting beta cell where its defining action is augmentation of glucose-induced insulin secretion. Upon GLP-1R activation, adenylyl cyclase (AC) is activated and cAMP is generated, leading, in turn, to cAMP-dependent activation of second messenger pathways, such as the protein kinase A (PKA) and Epac pathways. As well as short-term effects of enhancing glucose-induced insulin secretion, continuous GLP-1R activation also increases insulin synthesis, beta cell proliferation, and neogenesis. Although these latter effects cannot be currently monitored in humans, there are substantial improvements in glucose tolerance and increases in both first phase and plateau phase insulin secretory responses in T2DM patients treated with Ex-4. This review will focus on the effects resulting from GLP-1R activation in the pancreas.
Figures

References
-
- Abraham EJ, Leech CA, Lin JC, Zulewski H, Habener JF. Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producing cells. Endocrinology. 2002;143:3152–3161. - PubMed
-
- Abrahamsen N, Nishimura E. Regulation of glucagon and glucagon-like peptide-1 receptor messenger ribonucleic acid expression in cultured rat pancreatic islets by glucose, cyclic adenosine 3′,5′-monophosphate, and glucocorticoids. Endocrinology. 1995;136:1572–1578. - PubMed
-
- Ahren B, Pacini G, Foley JE, Schweizer A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care. 2005;28:1936–1940. - PubMed
-
- Al-Sabah S, Donnelly D. The positive charge at Lys-288 of the glucagon-like peptide-1 (GLP-1) receptor is important for binding the N-terminus of peptide agonists. FEBS Lett. 2003;553:342–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

