Effects of 5' untranslated region diversity on the posttranscriptional regulation of the human reduced folate carrier
- PMID: 17306382
- PMCID: PMC1963461
- DOI: 10.1016/j.bbaexp.2006.12.006
Effects of 5' untranslated region diversity on the posttranscriptional regulation of the human reduced folate carrier
Abstract
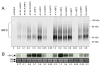
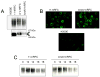
The human RFC (hRFC) gene is regulated by five major 5' non-coding exons, characterized by alternate transcription start sites and splice forms. The result is up to 14 hRFC transcripts for which different 5' untranslated regions (UTRs) are fused to a common coding sequence. By in vitro translation assays with hRFC constructs corresponding to the major transcript forms, most of the forms were translated poorly. Upon expression of the 5'UTR-hRFC constructs in hRFC-null HeLa cells, a range of steady state hRFC proteins and transcripts were detected that reflected relative transcript stabilities and, to a lesser extent, translation efficiencies. Transcripts including 5' UTRs derived from non-coding exon A encoded a modified hRFC protein translated from an upstream initiation site. When this modified hRFC protein was expressed in hRFC-null K562 cells, there were only minor differences in surface targeting, stability, or transport function from wild type hRFC. Our results demonstrate an important role for posttranscriptional determinants of cellular hRFC levels and activity.
Figures



Similar articles
-
Primary acute lymphoblastic leukemia cells use a novel promoter and 5'noncoding exon for the human reduced folate carrier that encodes a modified carrier translated from an upstream translational start.Clin Cancer Res. 2004 Aug 1;10(15):5111-22. doi: 10.1158/1078-0432.CCR-04-0116. Clin Cancer Res. 2004. PMID: 15297414
-
A humanized mouse model for the reduced folate carrier.Mol Genet Metab. 2008 Feb;93(2):95-103. doi: 10.1016/j.ymgme.2007.09.014. Epub 2007 Nov 5. Mol Genet Metab. 2008. PMID: 17983788 Free PMC article.
-
The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: identification of seven non-coding exons and characterization of a novel promoter.Biochem J. 2002 Nov 1;367(Pt 3):629-40. doi: 10.1042/BJ20020512. Biochem J. 2002. PMID: 12144527 Free PMC article.
-
Transcriptional regulation of the human reduced folate carrier in childhood acute lymphoblastic leukemia cells.Clin Cancer Res. 2006 Jan 15;12(2):608-16. doi: 10.1158/1078-0432.CCR-05-1954. Clin Cancer Res. 2006. PMID: 16428507
-
Roles of USF, Ikaros and Sp proteins in the transcriptional regulation of the human reduced folate carrier B promoter.Biochem J. 2004 Oct 15;383(Pt 2):249-57. doi: 10.1042/BJ20040414. Biochem J. 2004. PMID: 15214842 Free PMC article.
Cited by
-
The major facilitative folate transporters solute carrier 19A1 and solute carrier 46A1: biology and role in antifolate chemotherapy of cancer.Drug Metab Dispos. 2014 Apr;42(4):632-49. doi: 10.1124/dmd.113.055723. Epub 2014 Jan 6. Drug Metab Dispos. 2014. PMID: 24396145 Free PMC article. Review.
-
Oligomeric structure of the human reduced folate carrier: identification of homo-oligomers and dominant-negative effects on carrier expression and function.J Biol Chem. 2009 Jan 30;284(5):3285-3293. doi: 10.1074/jbc.M807206200. Epub 2008 Nov 19. J Biol Chem. 2009. PMID: 19019821 Free PMC article.
-
Identification of the minimal functional unit of the homo-oligomeric human reduced folate carrier.J Biol Chem. 2010 Feb 12;285(7):4732-40. doi: 10.1074/jbc.M109.086033. Epub 2009 Dec 17. J Biol Chem. 2010. PMID: 20018840 Free PMC article.
-
Folate transporter dynamics and therapy with classic and tumor-targeted antifolates.Sci Rep. 2021 Mar 18;11(1):6389. doi: 10.1038/s41598-021-85818-x. Sci Rep. 2021. PMID: 33737637 Free PMC article.
-
Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter.J Biol Chem. 2012 Feb 10;287(7):4982-95. doi: 10.1074/jbc.M111.306860. Epub 2011 Dec 16. J Biol Chem. 2012. PMID: 22179615 Free PMC article.
References
-
- Matherly LH, Goldman ID. Membrane transport of folates. Vitam Horm. 2003;66:404–456. - PubMed
-
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;47:7431–7457. - PubMed
-
- Flatley RM, Payton SG, Taub JW, Matherly LH. Primary acute lymphoblastic leukemia cells use a novel promoter and 5’non-coding exon for the human reduced folate carrier that encodes a modified carrier translated from an upstream translational start. Clin Cancer Res. 2004;10:5111–5222. Erratum in: 2005 Clin. Cancer Res. 20:7586. - PubMed
-
- Ayoubi TAY, van de Ven WJM. Regulation of gene expression by alternative promoters. FASEB J. 1996;10:453–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

