A killed, genetically engineered derivative of a wild-type extraintestinal pathogenic E. coli strain is a vaccine candidate
- PMID: 17306426
- PMCID: PMC1913199
- DOI: 10.1016/j.vaccine.2007.01.100
A killed, genetically engineered derivative of a wild-type extraintestinal pathogenic E. coli strain is a vaccine candidate
Abstract
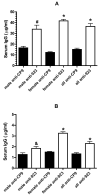
Infections due to extraintestinal pathogenic E. coli (ExPEC) result in significant morbidity, mortality and increased healthcare costs. An efficacious vaccine against ExPEC would be desirable. In this report, we explore the use of killed-whole E. coli as a vaccine immunogen. Given the diversity of capsule and O-antigens in ExPEC, we have hypothesized that alternative targets are viable vaccine candidates. We have also hypothesized that immunization with a genetically engineered strain that is deficient in the capsule and O-antigen will generate a greater immune response against antigens other than the capsular and O-antigen epitopes than a wild-type strain. Lastly, we hypothesize that mucosal immunization with killed E. coli has the potential to generate a significant immune response. In this study, we demonstrated that nasal immunization with a formalin-killed ExPEC derivative deficient in capsule and O-antigen results in a significantly greater overall humoral response compared to its wild-type derivative (which demonstrates that capsule and/or the O-antigen impede the development of an optimal humoral immune response) and a significantly greater immune response against non-capsular and O-antigen epitopes. These antibodies also bound to a subset of heterologous ExPEC strains and enhanced neutrophil-mediated bactericidal activity against the homologous and a heterologous strain. Taken together, these studies support the concept that formalin-killed genetically engineered ExPEC derivatives are whole cell vaccine candidates to prevent infections due to ExPEC.
Figures






References
-
- McBean M, Rajamani S. Increasing rates of hospitalization due to septicemia in the US elderly population, 1986-1997. J Infect Dis. 2001;183:596–603. - PubMed
-
- Gransden W, Eykyn S, Phillips I, Rowe B. Bacteremia due to Escherichia coli: A study of 861 episodes. Rev Infect Dis. 1990;12:1008–18. - PubMed
-
- Roberts F, Geere I, Coldman A. A three-year study of positive blood cultures, with emphasis on prognosis. Rev Infect Dis. 1991;13:34–46. - PubMed
-
- Vastag B. New vaccine decreases rate of nosocomial infections. JAMA. 2001;285:1565. - PubMed
-
- Siegman-Igra Y, Fourer B, Orni-Wasserlauf R, Golan Y, Noy A, Schwartz D, et al. Reappraisal of comminity-acquired bacteremia: A proposal of a new classification for the spectrum of acquisition of bacteremia. Clin Infect Dis. 2002;34:1431–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

