PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae
- PMID: 17307739
- PMCID: PMC2790426
- DOI: 10.1074/jbc.M611593200
PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae
Abstract
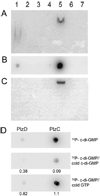
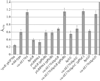
Cyclic diguanylate (c-di-GMP) is an allosteric activator and second messenger implicated in the regulation of a variety of biological processes in diverse bacteria. In Vibrio cholerae, c-di-GMP has been shown to inversely regulate biofilm-specific and virulence gene expression, suggesting that c-di-GMP signaling is important for the transition of V. cholerae from the environment to the host. However, the mechanism behind this regulation remains unknown. Recently, it was proposed that the PilZ protein domain represents a c-di-GMP-binding domain. Here we show that V. cholerae PilZ proteins bind c-di-GMP specifically and are involved in the regulation of biofilm formation, motility, and virulence. These findings confirm a role for PilZ proteins as c-di-GMP-sensing proteins within the c-di-GMP signaling network.
Figures



References
-
- Ross P, Aloni Y, Weinhouse H, Michaeli D, Weinberger-Ohana P, Mayer R, Benziman M. Carbohydr. Res. 1986;149:101–117.
-
- Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, Mayer R, Braun S, De Vroom E, Van Der Marel GA, Van Boom H, Benziman M. Nature. 1987;325:279–281. - PubMed
-
- Weinhouse H, Sapir S, Amikam D, Shilo Y, Volman G, Ohana P, Benziman M. FEBS Lett. 1997;416:207–211. - PubMed
-
- Drenkard E, Ausubel FM. Nature. 2002;416:740–743. - PubMed
-
- Garcia B, Latasa C, Solano C, Garcia-del Portillo F, Gamazo C, Lasa I. Mol. Microbiol. 2004;54:264–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

