Two-color far-field fluorescence nanoscopy
- PMID: 17307826
- PMCID: PMC1831704
- DOI: 10.1529/biophysj.107.104497
Two-color far-field fluorescence nanoscopy
Abstract
We demonstrate two-color fluorescence microscopy with nanoscale spatial resolution by applying stimulated emission depletion on fluorophores differing in their absorption and emission spectra. Green- and red-emitting fluorophores are selectively excited and quenched using dedicated beam pairs. The stimulated emission depletion beams deliver a lateral resolution of <30 nm and 65 nm for the green and the red color channel, respectively. The approximately 5 nm alignment accuracy of the two images establishes the precision with which differently labeled proteins are correlated in space. Colocalized nanoscopy is demonstrated with endosomal protein patterns and by resolving nanoclusters of a mitochondrial outer membrane protein, Tom20, in relation with the F(1)F(0)ATP synthase. The joint improvement of resolution and colocalization demonstrates the emerging potential of far-field fluorescence nanoscopy to study the spatial organization of macromolecules in cells.
Figures
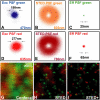
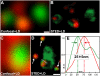

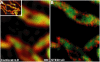
References
-
- Abbe, E. 1873. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Arch. f. Mikr. Anat. 9:413–420.
-
- Hell, S. W., and J. Wichmann. 1994. Breaking the diffraction resolution limit by stimulated emission: stimulated emission depletion microscopy. Opt. Lett. 19:780–782. - PubMed
-
- Hell, S. W. 2003. Toward fluorescence nanoscopy. Nat. Biotechnol. 21:1347–1355. - PubMed
-
- Westphal, V., and S. W. Hell. 2005. Nanoscale resolution in the focal plane of an optical microscope. Phys. Rev. Lett. 94:143903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

