Role of PBP1 in cell division of Staphylococcus aureus
- PMID: 17307860
- PMCID: PMC1855886
- DOI: 10.1128/JB.00044-07
Role of PBP1 in cell division of Staphylococcus aureus
Abstract
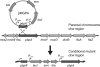
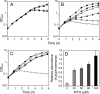
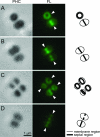
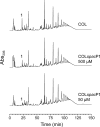
We constructed a conditional mutant of pbpA in which transcription of the gene was placed under the control of an IPTG (isopropyl-beta-D-thiogalactopyranoside)-inducible promoter in order to explore the role of PBP1 in growth, cell wall structure, and cell division. A methicillin-resistant strain and an isogenic methicillin-susceptible strain, each carrying the pbpA mutation, were unable to grow in the absence of the inducer. Conditional mutants of pbpA transferred into IPTG-free medium underwent a four- to fivefold increase in cell mass, which was not accompanied by a proportional increase in viable titer. Examination of thin sections of such cells by transmission electron microscopy or fluorescence microscopy of intact cells with Nile red-stained membranes showed a morphologically heterogeneous population of bacteria with abnormally increased sizes, distorted axial ratios, and a deficit in the number of cells with completed septa. Immunofluorescence with an antibody specific for PBP1 localized the protein to sites of cell division. No alteration in the composition of peptidoglycan was detectable in pbpA conditional mutants grown in the presence of a suboptimal concentration of IPTG, which severely restricted the rate of growth, and the essential function of PBP1 could not be replaced by PBP2A present in methicillin-resistant cells. These observations suggest that PBP1 is not a major contributor to the cross-linking of peptidoglycan and that its essential function must be intimately integrated into the mechanism of cell division.
Figures

References
-
- Berger-Bachi, B., A. Strassle, and F. H. Kayser. 1986. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur. J. Clin. Microbiol. 5:697-701. - PubMed
-
- Daniel, R. A., E. J. Harry, and J. Errington. 2000. Role of penicillin-binding protein PBP 2B in assembly and functioning of the division machinery of Bacillus subtilis. Mol. Microbiol. 35:299-311. - PubMed
-
- de Jonge, B. L., Y. S. Chang, D. Gage, and A. Tomasz. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J. Biol. Chem. 267:11248-11254. - PubMed
-
- de Lencastre, H., S. W. Wu, M. G. Pinho, A. M. Ludovice, S. Filipe, S. Gardete, R. Sobral, S. Gill, M. Chung, and A. Tomasz. 1999. Antibiotic resistance as a stress response: complete sequencing of a large number of chromosomal loci in Staphylococcus aureus strain COL that impact on the expression of resistance to methicillin. Microb. Drug Resist. 5:163-175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

