Dendritic cells with TGF-beta1 differentiate naive CD4+CD25- T cells into islet-protective Foxp3+ regulatory T cells
- PMID: 17307871
- PMCID: PMC1815265
- DOI: 10.1073/pnas.0611646104
Dendritic cells with TGF-beta1 differentiate naive CD4+CD25- T cells into islet-protective Foxp3+ regulatory T cells
Abstract
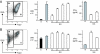
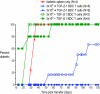
CD4(+)CD25(+)Foxp3(+) regulatory T cells (T regs) are important for preventing autoimmune diabetes and are either thymic-derived (natural) or differentiated in the periphery outside the thymus (induced). Here we show that beta-cell peptide-pulsed dendritic cells (DCs) from nonobese diabetic (NOD) mice can effectively induce CD4(+)CD25(+)Foxp3(+) T cells from naïve islet-specific CD4(+)CD25(-) T cells in the presence of TGF-beta1. These induced, antigen-specific T regs maintain high levels of clonotype-specific T cell receptor expression and exert islet-specific suppression in vitro. When cotransferred with diabetogenic cells into NOD scid recipients, T regs induced with DCs and TGF-beta1 prevent the development of diabetes. Furthermore, in overtly NOD mice, these cells are able to significantly protect syngeneic islet grafts from established destructive autoimmunity. These results indicate a role for DCs in the induction of antigen-specific CD4(+)CD25(+)Foxp3(+) T cells that can inhibit fully developed autoimmunity in a nonlymphopoenic host, providing an important potential strategy for immunotherapy in patients with autoimmune diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Upregulating CD4+CD25+FOXP3+ regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy.Transplantation. 2009 Jan 27;87(2):198-206. doi: 10.1097/TP.0b013e3181933261. Transplantation. 2009. PMID: 19155973
-
Dendritic cells with TGF-β1 and IL-2 differentiate naive CD4+ T cells into alloantigen-specific and allograft protective Foxp3+ regulatory T cells.Transplantation. 2012 Mar 27;93(6):580-8. doi: 10.1097/TP.0b013e318244dd67. Transplantation. 2012. PMID: 22270834 Free PMC article.
-
Rapamycin-conditioned donor dendritic cells differentiate CD4CD25Foxp3 T cells in vitro with TGF-beta1 for islet transplantation.Am J Transplant. 2010 Aug;10(8):1774-84. doi: 10.1111/j.1600-6143.2010.03199.x. Epub 2010 Jul 12. Am J Transplant. 2010. PMID: 20626386 Free PMC article.
-
Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes.Ann N Y Acad Sci. 2003 Apr;987:258-61. doi: 10.1111/j.1749-6632.2003.tb06057.x. Ann N Y Acad Sci. 2003. PMID: 12727648 Review.
-
Alterations in regulatory T-cells: rediscovered pathways in immunotoxicology.J Immunotoxicol. 2011 Oct-Dec;8(4):251-7. doi: 10.3109/1547691X.2011.598885. Epub 2011 Aug 17. J Immunotoxicol. 2011. PMID: 21848365 Free PMC article. Review.
Cited by
-
Current state of type 1 diabetes immunotherapy: incremental advances, huge leaps, or more of the same?Clin Dev Immunol. 2011;2011:432016. doi: 10.1155/2011/432016. Epub 2011 Jul 18. Clin Dev Immunol. 2011. PMID: 21785616 Free PMC article. Review.
-
Tuberculosis and Autoimmunity.Pathophysiology. 2022 Jun 13;29(2):298-318. doi: 10.3390/pathophysiology29020022. Pathophysiology. 2022. PMID: 35736650 Free PMC article. Review.
-
Local Immunomodulatory Strategies to Prevent Allo-Rejection in Transplantation of Insulin-Producing Cells.Adv Sci (Weinh). 2021 Sep;8(17):e2003708. doi: 10.1002/advs.202003708. Epub 2021 Jul 14. Adv Sci (Weinh). 2021. PMID: 34258870 Free PMC article. Review.
-
Excretory/secretory-products of Echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro.PLoS Negl Trop Dis. 2012;6(2):e1516. doi: 10.1371/journal.pntd.0001516. Epub 2012 Feb 21. PLoS Negl Trop Dis. 2012. PMID: 22363826 Free PMC article.
-
Infusion of UVB-treated splenic stromal cells induces suppression of beta cell antigen-specific T cell responses in NOD mice.J Autoimmun. 2008 Jun;30(4):283-92. doi: 10.1016/j.jaut.2007.11.017. Epub 2008 Jan 15. J Autoimmun. 2008. PMID: 18226498 Free PMC article.
References
-
- Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, et al. Immunity. 2005;23:115–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

