Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins
- PMID: 17307930
- PMCID: PMC1867334
- DOI: 10.1105/tpc.106.045351
Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins
Abstract
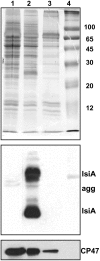
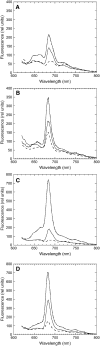
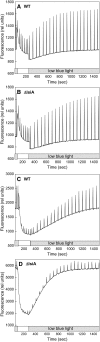
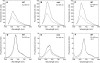
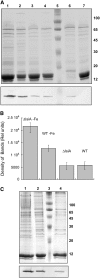
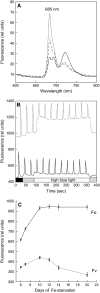
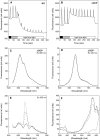
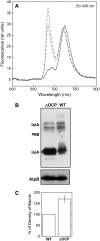
In response to iron deficiency, cyanobacteria synthesize the iron stress-induced chlorophyll binding protein IsiA. This protein protects cyanobacterial cells against iron stress. It has been proposed that the protective role of IsiA is related to a blue light-induced nonphotochemical fluorescence quenching (NPQ) mechanism. In iron-replete cyanobacterial cell cultures, strong blue light is known to induce a mechanism that dissipates excess absorbed energy in the phycobilisome, the extramembranal antenna of cyanobacteria. In this photoprotective mechanism, the soluble Orange Carotenoid Protein (OCP) plays an essential role. Here, we demonstrate that in iron-starved cells, blue light is unable to quench fluorescence in the absence of the phycobilisomes or the OCP. By contrast, the absence of IsiA does not affect the induction of fluorescence quenching or its recovery. We conclude that in cyanobacteria grown under iron starvation conditions, the blue light-induced nonphotochemical quenching involves the phycobilisome OCP-related energy dissipation mechanism and not IsiA. IsiA, however, does seem to protect the cells from the stress generated by iron starvation, initially by increasing the size of the photosystem I antenna. Subsequently, the IsiA converts the excess energy absorbed by the phycobilisomes into heat through a mechanism different from the dynamic and reversible light-induced NPQ processes.
Figures




References
-
- Adamska, I. (1997). Elips: Light-induced stress proteins. Physiol. Plant 100 794–805.
-
- Adamska, I. (2001). The Elip family of stress proteins in the thylakoids membranes of pro- and eukaryota. In Advances in Photosynthesis and Respiration-Regulation of Photosynthesis, Vol. 11, E.M. Aro and B. Andersson, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 487–505.
-
- Adir, N. (2005). Elucidation of the molecular structures of components of the phycobilisome: Reconstructing a giant. Photosynth. Res. 85 15–32. - PubMed
-
- Ajlani, G., and Vernotte, C. (1998). Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol. Biol. 37 577–580. - PubMed
-
- Ajlani, G., Vernotte, C., DiMagno, L., and Haselkorn, R. (1995). Phycobilisome core mutants of Synechocystis PCC 6803. Biochim. Biophys. Acta 1231 189–196.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

