Identification of a prepore large-complex stage in the mechanism of action of Clostridium perfringens enterotoxin
- PMID: 17307943
- PMCID: PMC1865780
- DOI: 10.1128/IAI.01737-06
Identification of a prepore large-complex stage in the mechanism of action of Clostridium perfringens enterotoxin
Abstract
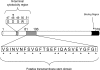
Clostridium perfringens enterotoxin (CPE) is the etiological agent of the third most common food-borne illness in the United States. The enteropathogenic effects of CPE result from formation of large CPE-containing complexes in eukaryotic cell membranes. Formation of these approximately 155- and approximately 200-kDa complexes coincides with plasma membrane permeability changes in eukaryotic cells, causing a Ca2+ influx that drives cell death pathways. CPE contains a stretch of amino acids (residues 81 to 106) that alternates markedly in side chain polarity (a pattern shared by the transmembrane domains of the beta-barrel pore-forming toxin family). The goal of this study, therefore, was to investigate whether this CPE region is involved in pore formation. Complete deletion of the CPE region from 81 to 106 produced a CPE variant that was noncytotoxic for Caco-2 cells and was unable to form CPE pores. However, this variant maintained the ability to form the approximately 155-kDa large complex. This large complex appears to be a prepore present on the plasma membrane surface since it showed greater susceptibility to proteases, increased complex instability, and a higher degree of dissociation from membranes compared to the large complex formed by recombinant CPE. When a D48A mutation was engineered into this prepore-forming CPE variant, the resultant variant was unable to form any prepore approximately 155-kDa large complex. Collectively these findings reveal a new step in CPE action, whereby receptor binding is followed by formation of a prepore large complex, which then inserts into membranes to form a pore.
Figures







Similar articles
-
Cysteine-scanning mutagenesis supports the importance of Clostridium perfringens enterotoxin amino acids 80 to 106 for membrane insertion and pore formation.Infect Immun. 2012 Dec;80(12):4078-88. doi: 10.1128/IAI.00069-12. Epub 2012 Sep 10. Infect Immun. 2012. PMID: 22966051 Free PMC article.
-
Identification of a Clostridium perfringens enterotoxin region required for large complex formation and cytotoxicity by random mutagenesis.Infect Immun. 1999 Nov;67(11):5634-41. doi: 10.1128/IAI.67.11.5634-5641.1999. Infect Immun. 1999. PMID: 10531210 Free PMC article.
-
Fine mapping of the N-terminal cytotoxicity region of Clostridium perfringens enterotoxin by site-directed mutagenesis.Infect Immun. 2004 Dec;72(12):6914-23. doi: 10.1128/IAI.72.12.6914-6923.2004. Infect Immun. 2004. PMID: 15557612 Free PMC article.
-
Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications.Toxins (Basel). 2016 Mar 16;8(3):73. doi: 10.3390/toxins8030073. Toxins (Basel). 2016. PMID: 26999202 Free PMC article. Review.
-
Clostridium perfringens enterotoxin acts by producing small molecule permeability alterations in plasma membranes.Toxicology. 1994 Feb 28;87(1-3):43-67. doi: 10.1016/0300-483x(94)90154-6. Toxicology. 1994. PMID: 8160188 Review.
Cited by
-
Evidence for a prepore stage in the action of Clostridium perfringens epsilon toxin.PLoS One. 2011;6(7):e22053. doi: 10.1371/journal.pone.0022053. Epub 2011 Jul 11. PLoS One. 2011. PMID: 21814565 Free PMC article.
-
Tight junctions, but not too tight: fine control of lung permeability by claudins.Am J Physiol Lung Cell Mol Physiol. 2009 Aug;297(2):L217-8. doi: 10.1152/ajplung.00196.2009. Epub 2009 Jun 12. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19525389 Free PMC article. Review. No abstract available.
-
Innovative and Highly Sensitive Detection of Clostridium perfringens Enterotoxin Based on Receptor Interaction and Monoclonal Antibodies.Toxins (Basel). 2021 Apr 8;13(4):266. doi: 10.3390/toxins13040266. Toxins (Basel). 2021. PMID: 33917845 Free PMC article.
-
Mechanism of Clostridium perfringens enterotoxin interaction with claudin-3/-4 protein suggests structural modifications of the toxin to target specific claudins.J Biol Chem. 2012 Jan 13;287(3):1698-708. doi: 10.1074/jbc.M111.312165. Epub 2011 Nov 28. J Biol Chem. 2012. PMID: 22128179 Free PMC article.
-
Characterizing the Contributions of Various Clostridium perfringens Enterotoxin Properties to In Vivo and In Vitro Permeability Effects.mSphere. 2022 Oct 26;7(5):e0027622. doi: 10.1128/msphere.00276-22. Epub 2022 Sep 7. mSphere. 2022. PMID: 36069435 Free PMC article.
References
-
- Chakrabarti, G., and B. A. McClane. 2005. The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell. Microbiol. 7:129-146. - PubMed
-
- Cheley, S., M. S. Malghani, L. Song, M. Hobaugh, J. E. Gouaux, J. Yang, and H. Bayley. 1997. Spontaneous oligomerization of a staphylococcal alpha-hemolysin conformationally constrained by removal of residues that form the transmembrane beta-barrel. Protein Eng. 10:1433-1443. - PubMed
-
- Deleage, G., and B. Roux. 1987. An algorithm for protein secondary structure prediction based on class prediction. Protein Eng. 1:289-294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

