Candida albicans and Candida dubliniensis respond differently to echinocandin antifungal agents in vitro
- PMID: 17307974
- PMCID: PMC1855534
- DOI: 10.1128/AAC.01525-06
Candida albicans and Candida dubliniensis respond differently to echinocandin antifungal agents in vitro
Abstract
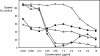
Candida dubliniensis isolates tested for susceptibility to anidulafungin, caspofungin, and micafungin commonly showed artifactual regrowth and/or trailing effects with MIC tests done under conditions involving a high initial yeast concentration. The artifacts were less common with Candida albicans and seldom seen for either species under Clinical and Laboratory Standards Institute method M27-A test conditions.
Figures
References
-
- Arthington-Skaggs, B. A., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock for the Candidemia Active Surveillance Group. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 462477-2481. - PMC - PubMed
-
- Chandrasekar, P. H., and J. D. Sobel. 2006. Micafungin: a new echinocandin. Clin. Infect. Dis. 421171-1178. - PubMed
-
- CLSI. 2006. Quality control minimal inhibitory concentration (MIC) limits for broth microdilution and MIC interpretive breakpoints; informational supplement, 2nd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, and J. L. Rodriguez-Tudela. 2005. Correlation between the procedure for antifungal susceptibility testing for Candida spp. of the European Committee on Antibiotic Susceptibility Testing (EUCAST) and four commercial techniques. Clin. Microbiol. Infect. 11486-492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

