Role of acidic pH in the susceptibility of intraphagocytic methicillin-resistant Staphylococcus aureus strains to meropenem and cloxacillin
- PMID: 17307986
- PMCID: PMC1855560
- DOI: 10.1128/AAC.01192-06
Role of acidic pH in the susceptibility of intraphagocytic methicillin-resistant Staphylococcus aureus strains to meropenem and cloxacillin
Abstract
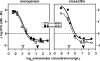
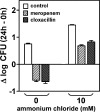
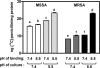
Early studies showed that methicillin-resistant Staphylococcus aureus (MRSA) strains are susceptible to beta-lactams when they are exposed to pH < or = 5.5 in broth. Because S. aureus survives in the phagolysosomes of macrophages, where the pH may be acidic, we have examined the susceptibility of MRSA ATCC 33591 phagocytized by human THP-1 macrophages to meropenem (MEM) and cloxacillin (CLX). Using a pharmacodynamic model assessing key pharmacological (50% effective concentration and maximal efficacy) and microbiological (static concentration) descriptors of antibiotic activity, we show that intraphagocytic MRSA strains are as sensitive to MEM and CLX as methicillin-susceptible S. aureus (MSSA; ATCC 25923). This observation was replicated in broth if the pH was brought to 5.5 and was confirmed with clinical strains. Electron microscopy showed that both the MRSA and the MSSA strains localized and multiplied in membrane-bounded structures (phagolysosomes) in the absence of beta-lactams. Incubation of the infected macrophages with ammonium chloride (to raise the phagolysosomal pH) made MRSA insensitive to MEM and CLX. No difference was seen in mec, mecA, mecI, mecR1, femA, and femB expression (reversed transcription-PCR) or in PBP 2a content (immunodetection) in MRSA grown in broth at pH 5.5 compared with that in MRSA grown in broth at 7.4. The level of [(14)C]benzylpenicillin binding to cell walls prepared from a non-beta-lactamase-producing MRSA clinical isolate was two times lower than that to cell walls prepared from MSSA ATCC 25923 at pH 7.4, but the levels increased to similar values for both strains at pH 5.5. These data suggest that the restoration of susceptibility of intraphagocytic of MRSA to MEM and CLX is due to the acidic pH prevailing in phagolysosomes and is mediated by an enhanced binding to penicillin-binding proteins.
Figures



References
-
- Dikstein, S., and A. Zlotogorski. 1994. Measurement of skin pH. Acta Dermatol. Venereol. Suppl. (Stockholm) 18518-20. - PubMed
-
- Foster, T. J. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3948-958. - PubMed
-
- Fuda, C., D. Hesek, M. Lee, W. Heilmayer, R. Novak, S. B. Vakulenko, and S. Mobashery. 2006. Mechanistic basis for the action of new cephalosporin antibiotics effective against methicillin- and vancomycin-resistant Staphylococcus aureus. J. Biol. Chem. 28110035-10041. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

