In vivo pharmacodynamic activity of the glycopeptide dalbavancin
- PMID: 17307987
- PMCID: PMC1855559
- DOI: 10.1128/AAC.01264-06
In vivo pharmacodynamic activity of the glycopeptide dalbavancin
Abstract
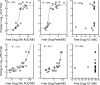
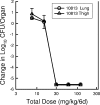
Dalbavancin is a lipoglycopeptide antibiotic with broad-spectrum activity against gram-positive cocci and a markedly prolonged serum elimination half-life. We used the neutropenic murine thigh and lung infection models to characterize the pharmacodynamics of dalbavancin. Single-dose pharmacokinetic studies demonstrated linear kinetics and a prolonged elimination half-life which ranged from 7.6 to 13.1 h over the dose range of 2.5 to 80 mg/kg of body weight. The level of protein binding in mouse serum was 98.4%. The time course of in vivo activity of dalbavancin over the same dose range was examined in neutropenic ICR Swiss mice infected with a strain of either Streptococcus pneumoniae or Staphylococcus aureus by using the thigh infection model. The burden of organisms for S. pneumoniae was markedly reduced over the initial 24 h of study, and organism regrowth was suppressed in a dose-dependent fashion for up to the entire 96 h of study following dalbavancin doses of 2.5 mg/kg or greater. Dalbavancin doses of 20 mg/kg or greater resulted in less killing of S. aureus but were still followed by a prolonged suppression of regrowth. Multiple-dosing-regimen studies with the same organisms were used to determined which of the pharmacodynamic indices (maximum concentration in serum [C(max)]/MIC, area under the concentration-versus-time curve [AUC]/MIC, or the duration of time that levels in serum exceed the MIC) best correlated with treatment efficacy. These studies used a dose range of 3.8 to 480 mg/kg/6 days fractionated into 2, 4, 6, or 12 doses over the 144-h dosing period. Nonlinear regression analysis was used to examine the data fit with each pharmacodynamic index. Dalbavancin administration by the use of large, widely spaced doses was the most efficacious for both organisms. Both the 24-h AUC/MIC and the C(max)/MIC parameters correlated well with the in vivo efficacy of treatment against S. pneumoniae and S. aureus (for 24-h AUC/MIC, R(2) = 78 and 77%, respectively; for C(max)/MIC, R(2) = 90 and 57%, respectively). The free-drug 24-h AUC/MICs required for a bacteriostatic effect were 17 +/- 7 for five S. pneumoniae isolates. A similar treatment endpoint for the treatment against five strains of S. aureus required a larger dalbavancin exposure, with a mean free-drug 24-h AUC/MIC of 265 +/- 143. Beta-lactam resistance did not affect the pharmacodynamic target. The dose-response curves were relatively steep for both species; thus, the pharmacodynamic target needed to achieve organism reductions of 1 or 2 log(10) in the mice were not appreciably larger (1.3- to 1.6-fold). Treatment was similarly efficacious in neutropenic mice and in the lung infection model. The dose-dependent efficacy and prolonged elimination half-life of dalbavancin support the widely spaced regimens used in clinical trials. The free-drug 24-h AUC/MIC targets identified in these studies should be helpful for discerning rational susceptibility breakpoints. The current MIC(90) for the target gram-positive organisms would fall within this value.
Figures

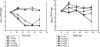
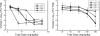
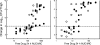
References
-
- Andes, D., and W. A. Craig. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19261-268. - PubMed
-
- Buckwalter, M., and J. A. Dowell. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipopeptide. J. Clin. Pharmacol. 451279-1287. - PubMed
-
- Chambers, H. F. 2005. Community-associated MRSA—resistance and virulence converge. N. Engl. J. Med. 3521485-1487. - PubMed
-
- Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 261-12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

