New insights into microbial oxidation of antimony and arsenic
- PMID: 17308197
- PMCID: PMC1855643
- DOI: 10.1128/AEM.02789-06
New insights into microbial oxidation of antimony and arsenic
Abstract
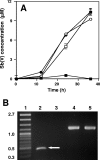
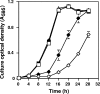
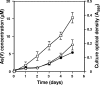
Sb(III) oxidation was documented in an Agrobacterium tumefaciens isolate that can also oxidize As(III). Equivalent Sb(III) oxidation rates were observed in the parental wild-type organism and in two well-characterized mutants that cannot oxidize As(III) for fundamentally different reasons. Therefore, despite the literature suggesting that Sb(III) and As(III) may be biochemical analogs, Sb(III) oxidation is catalyzed by a pathway different than that used for As(III). Sb(III) and As(III) oxidation was also observed for an eukaryotic acidothermophilic alga belonging to the order Cyanidiales, implying that the ability to oxidize metalloids may be phylogenetically widespread.
Figures

Similar articles
-
Microbial Antimony Biogeochemistry: Enzymes, Regulation, and Related Metabolic Pathways.Appl Environ Microbiol. 2016 Aug 30;82(18):5482-95. doi: 10.1128/AEM.01375-16. Print 2016 Sep 15. Appl Environ Microbiol. 2016. PMID: 27342551 Free PMC article. Review.
-
Arsenite oxidase also functions as an antimonite oxidase.Appl Environ Microbiol. 2015 Mar;81(6):1959-65. doi: 10.1128/AEM.02981-14. Epub 2015 Jan 9. Appl Environ Microbiol. 2015. PMID: 25576601 Free PMC article.
-
Abiotic and biotic factors responsible for antimonite oxidation in Agrobacterium tumefaciens GW4.Sci Rep. 2017 Mar 2;7:43225. doi: 10.1038/srep43225. Sci Rep. 2017. PMID: 28252030 Free PMC article.
-
Regulation of antimonite oxidation and resistance by the phosphate regulator PhoB in Agrobacterium tumefaciens GW4.Microbiol Res. 2019 Sep;226:10-18. doi: 10.1016/j.micres.2019.04.008. Epub 2019 Apr 25. Microbiol Res. 2019. PMID: 31284939
-
[Bacteria live on arsenic analysis of microbial arsenic metabolism--a review].Wei Sheng Wu Xue Bao. 2011 Feb;51(2):154-60. Wei Sheng Wu Xue Bao. 2011. PMID: 21574375 Review. Chinese.
Cited by
-
A Critical Review of Resistance and Oxidation Mechanisms of Sb-Oxidizing Bacteria for the Bioremediation of Sb(III) Pollution.Front Microbiol. 2021 Sep 7;12:738596. doi: 10.3389/fmicb.2021.738596. eCollection 2021. Front Microbiol. 2021. PMID: 34557178 Free PMC article. Review.
-
Global Regulator IscR Positively Contributes to Antimonite Resistance and Oxidation in Comamonas testosteroni S44.Front Mol Biosci. 2015 Dec 18;2:70. doi: 10.3389/fmolb.2015.00070. eCollection 2015. Front Mol Biosci. 2015. PMID: 26734615 Free PMC article.
-
Metagenomic approach reveals variation of microbes with arsenic and antimony metabolism genes from highly contaminated soil.PLoS One. 2014 Oct 9;9(10):e108185. doi: 10.1371/journal.pone.0108185. eCollection 2014. PLoS One. 2014. PMID: 25299175 Free PMC article.
-
Synergistic Impacts of Arsenic and Antimony Co-contamination on Diazotrophic Communities.Microb Ecol. 2022 Jul;84(1):44-58. doi: 10.1007/s00248-021-01824-6. Epub 2021 Aug 16. Microb Ecol. 2022. PMID: 34398256
-
Microbial Antimony Biogeochemistry: Enzymes, Regulation, and Related Metabolic Pathways.Appl Environ Microbiol. 2016 Aug 30;82(18):5482-95. doi: 10.1128/AEM.01375-16. Print 2016 Sep 15. Appl Environ Microbiol. 2016. PMID: 27342551 Free PMC article. Review.
References
-
- Allen, M. B. 1959. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch. Microbiol. 32:270-277. - PubMed
-
- Andreae, M. O., J.-F. Asmodé, P. Foster, and L. Van't Dack. 1981. Determination of antimony(III), antimony(V), and methylantimony species in natural waters by atomic absorption spectrometry with hydride generation. Anal. Chem. 53:1766-1771.
-
- Andrews, P., W. R. Cullen, and E. Polishchuk. 2000. Arsenic and antimony biomethylation by Scopulariopsis brevicaulis: interaction of arsenic and antimony compounds. Environ. Sci. Technol. 34:2249-2253.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

