In vivo mechanical loading modulates insulin-like growth factor binding protein-2 gene expression in rat osteocytes
- PMID: 17308996
- PMCID: PMC1914289
- DOI: 10.1007/s00223-006-0077-4
In vivo mechanical loading modulates insulin-like growth factor binding protein-2 gene expression in rat osteocytes
Abstract
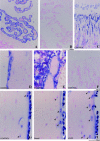
Mechanical stimulation is essential for maintaining skeletal integrity. Mechanosensitive osteocytes are important during the osteogenic response. The growth hormone-insulin-like growth factor (GH-IGF) axis plays a key role during regulation of bone formation and remodeling. Insulin-like growth factor binding proteins (IGFBPs) are able to modulate IGF activity. The aim of this study was to characterize the role of IGFBP-2 in the translation of mechanical stimuli into bone formation locally in rat tibiae. Female Wistar rats were assigned to three groups (n = 5): load, sham, and control. The four-point bending model was used to induce a single period of mechanical loading on the tibial shaft. The effect on IGFBP-2 mRNA expression 6 hours after stimulation was determined with nonradioactive in situ hybridization on decalcified tibial sections. Endogenous IGFBP-2 mRNA was expressed in trabecular and cortical osteoblasts, some trabecular and subendocortical osteocytes, intracortical endothelial cells of blood vessels, and periosteum. Megakaryocytes, macrophages, and myeloid cells also expressed IGFBP-2 mRNA. Loading and sham loading did not affect IGFBP-2 mRNA expression in osteoblasts, bone marrow cells, and chondrocytes. An increase of IGFBP-2 mRNA-positive osteocytes was shown in loaded (1.68-fold) and sham-loaded (1.35-fold) endocortical tibial shaft. In conclusion, 6 hours after a single loading session, the number of IGFBP-2 mRNA-expressing osteocytes at the endosteal side of the shaft and inner lamellae was increased in squeezed and bended tibiae. Mechanical stimulation modulates IGFBP-2 mRNA expression in endocortical osteocytes. We suggest that IGFBP-2 plays a role in the lamellar bone formation process.
Figures
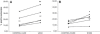
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S8756-3282(98)00090-8', 'is_inner': False, 'url': 'https://doi.org/10.1016/s8756-3282(98)00090-8'}, {'type': 'PubMed', 'value': '9737355', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9737355/'}]}
- Forwood MR, Bennett MB, Blowers AR, Nadorfi RL (1998) Modification of the in vivo four-point loading model for studying mechanically induced bone adaptation. Bone 23:307–310 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '8154314', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8154314/'}]}
- Turner CH, Forwood MR, Rho JY, Yoshikawa T (1994) Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res 9:87–97 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '8447384', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8447384/'}]}
- Yeh JK, Liu CC, Aloia JF (1993) Effects of exercise and immobilization on bone formation and resorption in young rats. Am J Physiol 264:E182–E189 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/BF00357631', 'is_inner': False, 'url': 'https://doi.org/10.1007/bf00357631'}, {'type': 'PubMed', 'value': '8299600', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8299600/'}]}
- Smith R, Rutherford OM (1993) Spine and total body bone mineral density and serum testosterone levels in male athletes. Eur J Appl Physiol Occup Physiol 67:330–334 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '7934763', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7934763/'}]}
- Hamdy RC, Anderson JS, Whalen KE, Harvill LM (1994) Regional differences in bone density of young men involved in different exercises. Med Sci Sports Exerc 26:884–888 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

