Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain
- PMID: 17309135
- PMCID: PMC2080505
Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain
Abstract
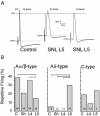
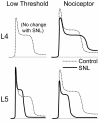
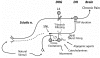
The pathogenesis of neuropathic pain is incompletely understood and treatments are often inadequate. Cytoplasmic Ca(2+) regulates numerous cellular processes in neurons. This review therefore examines the pathogenic contribution of altered inward Ca(2+) flux (I(Ca)) through voltage-gated Ca(2+) channels in sensory neurons after peripheral nerve injury. We reviewed studies that recorded membrane currents through intracellular and patch-clamp techniques, as well as intracellular Ca(2+) levels using fluorimetric indicators, and performed behavioral analysis of rodent nerve injury models. Following nerve injury by partial ligation, a response characterized by sustained lifting, shaking, and licking of the paw after sharp mechanical stimulation is a reliable indicator or neuropathic pain. Primary sensory neurons isolated from animals with this behavior show a decrease in high-voltage activated I(Ca) by approximately one third. Low voltage-activated I(Ca) is nearly eliminated by peripheral nerve injury. Loss of I(Ca) leads to decreased activation of Ca(2+)-activated K(+) currents, which are also directly reduced in traumatized neurons. As a result of these changes in membrane currents, membrane voltage recordings show increased action potential duration and diminished afterhyperpolarization. Excitability is elevated, as indicated by resting membrane potential depolarization and a decreased current threshold for action potential initiation. Traumatized nociceptive neurons develop increased repetitive firing during sustained depolarization after axotomy. Concurrently, cytoplasmic Ca(2+) transients are diminished. In conclusions, axotomized neurons, especially pain-conducting ones, develop instability and elevated excitability after peripheral injury. Treatment of neuronal I(Ca) loss at the level of injury of the dorsal root ganglion may provide a novel therapeutic pathway.
Figures

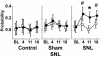

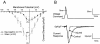
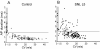
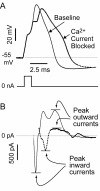
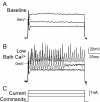
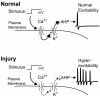
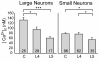
Similar articles
-
Painful nerve injury shortens the intracellular Ca2+ signal in axotomized sensory neurons of rats.Anesthesiology. 2007 Jul;107(1):106-16. doi: 10.1097/01.anes.0000267538.72900.68. Anesthesiology. 2007. PMID: 17585222 Free PMC article.
-
Contribution of calcium channel subtypes to the intracellular calcium signal in sensory neurons: the effect of injury.Anesthesiology. 2007 Jul;107(1):117-27. doi: 10.1097/01.anes.0000267511.21864.93. Anesthesiology. 2007. PMID: 17585223 Free PMC article.
-
Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats.Anesthesiology. 2005 Aug;103(2):360-76. doi: 10.1097/00000542-200508000-00020. Anesthesiology. 2005. PMID: 16052119
-
Painful channels in sensory neurons.Mol Cells. 2005 Dec 31;20(3):315-24. Mol Cells. 2005. PMID: 16404144 Review.
-
Neuropathic pain and injured nerve: peripheral mechanisms.Br Med Bull. 1991 Jul;47(3):619-30. doi: 10.1093/oxfordjournals.bmb.a072496. Br Med Bull. 1991. PMID: 1794075 Review.
Cited by
-
Calcium Signalling in Breast Cancer Associated Bone Pain.Int J Mol Sci. 2022 Feb 8;23(3):1902. doi: 10.3390/ijms23031902. Int J Mol Sci. 2022. PMID: 35163823 Free PMC article. Review.
-
Serotonin differentially modulates Ca2+ transients and depolarization in a C. elegans nociceptor.J Neurophysiol. 2015 Feb 15;113(4):1041-50. doi: 10.1152/jn.00665.2014. Epub 2014 Nov 19. J Neurophysiol. 2015. PMID: 25411461 Free PMC article.
-
Effects of familial hemiplegic migraine type 1 mutation T666M on voltage-gated calcium channel activities in trigeminal ganglion neurons.J Neurophysiol. 2012 Mar;107(6):1666-80. doi: 10.1152/jn.00551.2011. Epub 2011 Dec 21. J Neurophysiol. 2012. PMID: 22190617 Free PMC article.
-
Sensory neuron dysfunction in orthotopic mouse models of colon cancer.J Neuroinflammation. 2022 Aug 12;19(1):204. doi: 10.1186/s12974-022-02566-z. J Neuroinflammation. 2022. PMID: 35962398 Free PMC article.
-
IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain.Int J Mol Sci. 2022 Feb 15;23(4):2142. doi: 10.3390/ijms23042142. Int J Mol Sci. 2022. PMID: 35216258 Free PMC article.
References
-
- Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–85. - PubMed
-
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–82. - PubMed
-
- Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42:205–13. - PubMed
-
- Mao J, Price DD, Coghill RC, Mayer DJ, Hayes RL. Spatial patterns of spinal cord [14C]-2-deoxyglucose metabolic activity in a rat model of painful peripheral mononeuropathy. Pain. 1992;50:89–100. - PubMed
-
- Laird JM, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. J Neurophysiol. 1993;69:2072–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous