Biosynthesis of thiamin thiazole in eukaryotes: conversion of NAD to an advanced intermediate
- PMID: 17309261
- PMCID: PMC2536526
- DOI: 10.1021/ja067606t
Biosynthesis of thiamin thiazole in eukaryotes: conversion of NAD to an advanced intermediate
Abstract
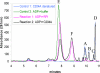
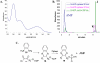
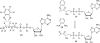
Thiazole synthase catalyzes the formation of the thiazole moiety of thiamin pyrophosphate. The enzyme from Saccharomyces cerevisiae (THI4) copurifies with a set of strongly bound adenylated metabolites. One of them has been characterized as the ADP adduct of 5-(2-hydroxyethyl)-4-methylthiazole-2-carboxylic acid. Attempts toward yielding active wild-type THI4 by releasing protein-bound metabolites have failed so far. Here, we describe the identification and characterization of two partially active mutants (C204A and H200N) of THI4. Both mutants catalyzed the release of the nicotinamide moiety from NAD to produce ADP-ribose, which was further converted to ADP-ribulose. In the presence of glycine, both the mutants catalyzed the formation of an advanced intermediate. The intermediate was trapped with ortho-phenylenediamine, yielding a stable quinoxaline derivative, which was characterized by NMR spectroscopy and ESI-MS. These observations confirm NAD as the substrate for THI4 and elucidate the early steps of this unique biosynthesis of the thiazole moiety of thiamin in eukaryotes.
Figures









References
-
- Butterworth RF. Thiamin deficiency and brain disorders. Nutr. Res. Rev. 2003;16(2):277–283. - PubMed
-
- Jordan F. Current mechanistic understanding of thiamin diphosphate-dependent enzymatic reactions. Nat. Prod. Rep. 2003;20(2):184–201. - PubMed
-
- Settembre E, Begley TP, Ealick SE. Structural biology of enzymes of the thiamin biosynthesis pathway. Curr. Opin. Struct. Biol. 2003;13(6):739–747. - PubMed
-
- Begley TP, Downs DM, Ealick SE, McLafferty FW, Van Loon APGM, Taylor S, Campobasso N, Chiu H-J, Kinsland C, Reddick JJ, Xi J. Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 1999;171(5):293–300. - PubMed
-
- Spenser ID, White RL. Biosynthesis of vitamin B1 (thiamin): an instance of biochemical diversity. Angew. Chem. Int. Ed. Engl. 1997;36(10):1032–1046.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

