Intraplantar PGE2 causes nociceptive behaviour and mechanical allodynia: the role of prostanoid E receptors and protein kinases
- PMID: 17310141
- PMCID: PMC2013868
- DOI: 10.1038/sj.bjp.0707149
Intraplantar PGE2 causes nociceptive behaviour and mechanical allodynia: the role of prostanoid E receptors and protein kinases
Abstract
Background and purpose: Receptor subtypes involved in PGE(2)-induced nociception are still controversial. The present study investigated the prostanoid E receptor (EP) subtypes and the protein kinase (PK) pathways involved in the nociception induced by PGE(2) injection in the mouse paw.
Experimental approach: Paw-licking and mechanical allodynia were measured in vivo and protein kinase activation ex vivo by Western blots of extracts of paw skin.
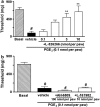
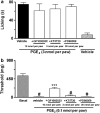
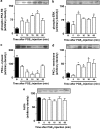
Key results: Intraplantar (i.pl.) injection of PGE(2) into the mouse paw caused nociceptive behaviour of short duration with mean ED(50) of 1.43 nmol. PGE(2) produced a longer-lasting mechanical allodynia, with an ED(50) of 0.05 nmol. Intraplantar injection of antagonists at EP(3) or EP(4), but not at EP(1) or EP(2) receptors inhibited PGE(2)-induced paw-licking. Paw-licking caused by PGE(2) was blocked by an inhibitor of PKA but only partially decreased by inhibition of the extracellular-regulated kinase (ERK). Selective inhibitors of PKC, c-Jun N-terminal kinase (JNK) or p38, all failed to affect PGE(2)-induced paw-licking. An EP(3) antagonist inhibited PGE(2)-induced mechanical allodynia. However, inhibitors of PKA, PKC or ERK, but not p38 or JNK, also partially inhibited PGE(2)-induced mechanical allodynia. Western blot analyses confirmed that i.pl. injection of PGE(2) activated PKA, PKCalpha, and mitogen activated kinases (MAPKs) in the paw. Co-treatment with EP(3) or EP(4) receptor antagonists reduced PGE(2)-induced PKA and ERK, but not PKCalpha activation.
Conclusions and implications: The present results indicate that the nociceptive behaviour and mechanical allodynia caused by i.pl. PGE(2) are mediated through activation of distinct EP receptors and PK-dependent mechanisms.
Figures





References
-
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. - PubMed
-
- André E, Ferreira J, Malheiros A, Yunes RA, Calixto JB. Evidence for the involvement of vanilloid receptor in the antinociception produced by the dialdeydes unsaturated sesquiterpenes polygodial and drimanial in rats. Neuropharmacology. 2004;46:590–597. - PubMed
-
- Burkey TH, Regan JW. Activation of mitogen-activated protein kinase by the human prostaglandin EP3A receptor. Biochem Biophys Res Commun. 1995;211:152–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

