Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors
- PMID: 17310245
- PMCID: PMC2227320
- DOI: 10.1038/nn1856
Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors
Abstract
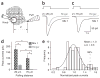
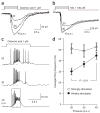
Most sensory systems are primarily specialized to detect one sensory modality. Here we report that olfactory sensory neurons (OSNs) in the mammalian nose can detect two distinct modalities transmitted by chemical and mechanical stimuli. As revealed by patch-clamp recordings, many OSNs respond not only to odorants, but also to mechanical stimuli delivered by pressure ejections of odor-free Ringer solution. The mechanical responses correlate directly with the pressure intensity and show several properties similar to those induced by odorants, including onset latency, reversal potential and adaptation to repeated stimulation. Blocking adenylyl cyclase or knocking out the cyclic nucleotide-gated channel CNGA2 eliminates the odorant and the mechanical responses, suggesting that both are mediated by a shared cAMP cascade. We further show that this mechanosensitivity enhances the firing frequency of individual neurons when they are weakly stimulated by odorants and most likely drives the rhythmic activity (theta oscillation) in the olfactory bulb to synchronize with respiration.
Figures



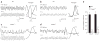
References
-
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. - PubMed
-
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. - PubMed
-
- Adrian ED. The role of air movement in olfactory stimulation. J Physiol (Lond) 1951;114:4–5p. - PubMed
-
- Macrides F, Chorover SL. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science. 1972;175:84–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

