Reporting methods of blinding in randomized trials assessing nonpharmacological treatments
- PMID: 17311468
- PMCID: PMC1800311
- DOI: 10.1371/journal.pmed.0040061
Reporting methods of blinding in randomized trials assessing nonpharmacological treatments
Abstract
Background: Blinding is a cornerstone of treatment evaluation. Blinding is more difficult to obtain in trials assessing nonpharmacological treatment and frequently relies on "creative" (nonstandard) methods. The purpose of this study was to systematically describe the strategies used to obtain blinding in a sample of randomized controlled trials of nonpharmacological treatment.
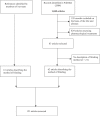
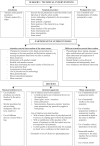
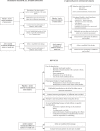
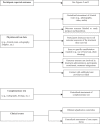
Methods and findings: We systematically searched in Medline and the Cochrane Methodology Register for randomized controlled trials (RCTs) assessing nonpharmacological treatment with blinding, published during 2004 in high-impact-factor journals. Data were extracted using a standardized extraction form. We identified 145 articles, with the method of blinding described in 123 of the reports. Methods of blinding of participants and/or health care providers and/or other caregivers concerned mainly use of sham procedures such as simulation of surgical procedures, similar attention-control interventions, or a placebo with a different mode of administration for rehabilitation or psychotherapy. Trials assessing devices reported various placebo interventions such as use of sham prosthesis, identical apparatus (e.g., identical but inactivated machine or use of activated machine with a barrier to block the treatment), or simulation of using a device. Blinding participants to the study hypothesis was also an important method of blinding. The methods reported for blinding outcome assessors relied mainly on centralized assessment of paraclinical examinations, clinical examinations (i.e., use of video, audiotape, photography), or adjudications of clinical events.
Conclusions: This study classifies blinding methods and provides a detailed description of methods that could overcome some barriers of blinding in clinical trials assessing nonpharmacological treatment, and provides information for readers assessing the quality of results of such trials.
Conflict of interest statement
Figures
References
-
- Gluud LL. Bias in clinical intervention research. Am J Epidemiol. 2006;163:493–501. - PubMed
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. - PubMed
-
- Schulz KF, Chalmers I, Altman DG. The landscape and lexicon of blinding in randomized trials. Ann Intern Med. 2002;136:254–259. - PubMed
-
- Devereaux PJ, Manns BJ, Ghali WA, Quan H, Lacchetti C, et al. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA. 2001;285:2000–2003. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

