Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors
- PMID: 17311675
- PMCID: PMC1851381
- DOI: 10.1186/bcr1656
Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors
Abstract
Background: Breast carcinoma is accompanied by changes in the acellular and cellular components of the microenvironment, the latter typified by a switch from fibroblasts to myofibroblasts.
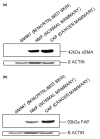
Methods: We utilised conditioned media cultures, Western blot analysis and immunocytochemistry to investigate the differential effects of normal mammary fibroblasts (NMFs) and mammary cancer-associated fibroblasts (CAFs) on the phenotype and behaviour of PMC42-LA breast cancer cells. NMFs were obtained from a mammary gland at reduction mammoplasty, and CAFs from a mammary carcinoma after resection.
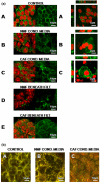
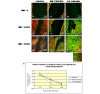
Results: We found greater expression of myofibroblastic markers in CAFs than in NMFs. Medium from both CAFs and NMFs induced novel expression of alpha-smooth muscle actin and cytokeratin-14 in PMC42-LA organoids. However, although conditioned media from NMFs resulted in distribution of vimentin-positive cells to the periphery of PMC42-LA organoids, this was not seen with CAF-conditioned medium. Upregulation of vimentin was accompanied by a mis-localization of E-cadherin, suggesting a loss of adhesive function. This was confirmed by visualizing the change in active beta-catenin, localized to the cell junctions in control cells/cells in NMF-conditioned medium, to inactive beta-catenin, localized to nuclei and cytoplasm in cells in CAF-conditioned medium.
Conclusion: We found no significant difference between the influences of NMFs and CAFs on PMC42-LA cell proliferation, viability, or apoptosis; significantly, we demonstrated a role for CAFs, but not for NMFs, in increasing the migratory ability of PMC42-LA cells. By concentrating NMF-conditioned media, we demonstrated the presence of factor(s) that induce epithelial-mesenchymal transition in NMF-conditioned media that are present at higher levels in CAF-conditioned media. Our in vitro results are consistent with observations in vivo showing that alterations in stroma influence the phenotype and behaviour of surrounding cells and provide evidence for a role for CAFs in stimulating cancer progression via an epithelial-mesenchymal transition. These findings have implications for our understanding of the roles of signalling between epithelial and stromal cells in the development and progression of mammary carcinoma.
Figures




Similar articles
-
Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells.Endocr Relat Cancer. 2013 Jan 7;20(1):1-12. doi: 10.1530/ERC-12-0227. Print 2013 Feb. Endocr Relat Cancer. 2013. PMID: 23111755
-
Myoepithelial molecular markers in human breast carcinoma PMC42-LA cells are induced by extracellular matrix and stromal cells.In Vitro Cell Dev Biol Anim. 2006 Nov-Dec;42(10):298-307. doi: 10.1290/0601004.1. In Vitro Cell Dev Biol Anim. 2006. PMID: 17316063
-
Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor gamma: mechanism of desmoplastic reaction.Cancer Res. 2001 Mar 1;61(5):2250-5. Cancer Res. 2001. PMID: 11280794
-
Carcinoma-associated fibroblasts: non-neoplastic tumour-promoting mesenchymal cells.J Cell Physiol. 2013 Aug;228(8):1651-7. doi: 10.1002/jcp.24347. J Cell Physiol. 2013. PMID: 23460038 Review.
-
Cancer-associated fibroblasts in digestive tumors.World J Gastroenterol. 2014 Dec 21;20(47):17804-18. doi: 10.3748/wjg.v20.i47.17804. World J Gastroenterol. 2014. PMID: 25548479 Free PMC article. Review.
Cited by
-
The contemporary role of renal mass biopsy in the management of small renal tumors.Front Oncol. 2012 Sep 10;2:106. doi: 10.3389/fonc.2012.00106. eCollection 2012. Front Oncol. 2012. PMID: 22973552 Free PMC article.
-
Direct Interactions With Cancer-Associated Fibroblasts Lead to Enhanced Pancreatic Cancer Stem Cell Function.Pancreas. 2019 Mar;48(3):329-334. doi: 10.1097/MPA.0000000000001249. Pancreas. 2019. PMID: 30747824 Free PMC article.
-
Interface between breast cancer cells and the tumor microenvironment using platelet-rich plasma to promote tumor angiogenesis - influence of platelets and fibrin bundles on the behavior of breast tumor cells.Oncotarget. 2017 Mar 7;8(10):16851-16874. doi: 10.18632/oncotarget.15170. Oncotarget. 2017. PMID: 28187434 Free PMC article.
-
Gene expression profiling of calcifications in breast cancer.Sci Rep. 2017 Sep 12;7(1):11427. doi: 10.1038/s41598-017-11331-9. Sci Rep. 2017. PMID: 28900139 Free PMC article.
-
Contribution of Fibroblast and Mast Cell (Afferent) and Tumor (Efferent) IL-6 Effects within the Tumor Microenvironment.Cancer Microenviron. 2012 Apr;5(1):83-93. doi: 10.1007/s12307-012-0098-7. Epub 2012 Feb 8. Cancer Microenviron. 2012. PMID: 22314376 Free PMC article.
References
-
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. - PubMed
-
- Gache C, Berthois Y, Martin PM, Saez S. Positive regulation of normal and tumoral mammary epithelial cell proliferation by fibroblasts in coculture. In Vitro Cell Dev Biol Anim. 1998;34:347–351. - PubMed
-
- Farber E. The multistep nature of cancer development. Cancer Res. 1984;44:4217–4223. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

