Temporal and epigenetic regulation of neurodevelopmental plasticity
- PMID: 17311782
- PMCID: PMC2605484
- DOI: 10.1098/rstb.2006.2010
Temporal and epigenetic regulation of neurodevelopmental plasticity
Abstract
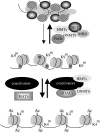
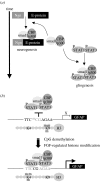
The anticipated therapeutic uses of neural stem cells depend on their ability to retain a certain level of developmental plasticity. In particular, cells must respond to developmental manipulations designed to specify precise neural fates. Studies in vivo and in vitro have shown that the developmental potential of neural progenitor cells changes and becomes progressively restricted with time. For in vitro cultured neural progenitors, it is those derived from embryonic stem cells that exhibit the greatest developmental potential. It is clear that both extrinsic and intrinsic mechanisms determine the developmental potential of neural progenitors and that epigenetic, or chromatin structural, changes regulate and coordinate hierarchical changes in fate-determining gene expression. Here, we review the temporal changes in developmental plasticity of neural progenitor cells and discuss the epigenetic mechanisms that underpin these changes. We propose that understanding the processes of epigenetic programming within the neural lineage is likely to lead to the development of more rationale strategies for cell reprogramming that may be used to expand the developmental potential of otherwise restricted progenitor populations.
Figures

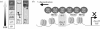

References
-
- Agarwala S, Sanders T.A, Ragsdale C.W. Sonic hedgehog control of size and shape in midbrain pattern formation. Science. 2001;291:2147–2150. - PubMed
-
- Aiba K, Sharov A.A, Carter M.G, Foroni C, Vescovi A.L, Ko M.S. Defining a developmental path to neural fate by global expression profiling of mouse embryonic stem cells and adult neural stem/progenitor cells. Stem Cells. 2005;4:889–895. - PubMed
-
- Ajamian F, Suuronen T, Salminen A, Reeben M. Upregulation of class II histone deacetylases mRNA during neural differentiation of cultured rat hippocampal progenitor cells. Neurosci. Lett. 2003;346:57–60. - PubMed
-
- Arney K.L, Fisher A.G. Epigenetic aspects of differentiation. J. Cell Sci. 2004;117:4355–4363. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

